* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Introduction Part1
Cooperative binding wikipedia , lookup
Circular dichroism wikipedia , lookup
Protein domain wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
List of types of proteins wikipedia , lookup
ATP-binding cassette transporter wikipedia , lookup
P-type ATPase wikipedia , lookup
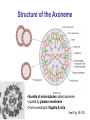
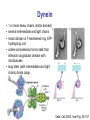
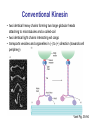
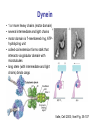
Motor Proteins - Introduction Part 1 Biochemistry 4000 Dr. Ute Kothe Motor Proteins Motor Proteins convert chemical energy into motion. • chemical energy is derived from ATP hydrolysis • motion is generated by conformational changes depending on the bound nucleotide Myosin Kinesin Dynein Motor Protein Function Myosin (18 known classes, 40 different myosins in humans) Movement along actin fibres • Muscle movement • Cytokinesis (cytoplasmic division, tightening of contractile ring) • Transport of cargo along microfilaments (vescicles etc.) Kinesin (16 classes) Movement along microtubule tracks, usually to (+) end • Transport of cargos: vesicles, organelles, cytosolic components such as mRNAs & proteins, chromosomes Dynein (12 mammalian dyneins) Movement along microtubule tracks, to (-) end, i.e. cell center • Cytoplasmic dyneins: transport of cargos such as vesicles • Axonemal dyneins: Movement of cilia and flagella Tubulin • building block of microtubules • heterodimer of closely related Tubulin a &b Tubulin b + GDP • G proteins: N-terminal residues fold into G domain-like structure • a-tubulin’s GTP buried at subunit interface, nonexchangable, not hydrolyzed • b-tubulin’s GTP is solvent exposed until tubulin dimers polymerize Tubulin a + GTP • upon polymerization, a-tubulin from adjacent dimer provides catalytic residue to hydrolyze b-tubulin’s GTP; resulting GDP is nonexchangeable unless tubulin dissociates from microtubule Voet Fig. 35-89 Microtubules 1. 2. 3. 4. Tubulins interact head to tail to form a long protofilament Protofilaments align side by side in curved sheet Sheet of 13 (9-16) protomers closes on itself to form microtubule Microtubule lengthens by addition of tubulins to both ends (preferentially to + end, i.e. the end terminating in b-tubulins) Voet Fig. 35-92 Structure of the Axoneme • Bundle of microtubules called axoneme • coated by plasma membrane • Forms eukaroytic flagella & cilia Voet Fig. 35-102 Dynein • 1 or more heavy chains (motor domain) • several intermediate and light chains • motor domain is 7-membered ring, ATPhydrolyzing unit • coiled-coil extension forms stalk that interacts via globular domain with microtubules • long stem (with intermediate and light chains) binds cargo Valle, Cell 2003; Voet Fig. 35-107 Conventional Kinesin • two identical heavy chains forming two large globular heads attaching to microtubules and a coiled-coil • two identical light chains interacting wit cargo • transports vesicles and organelles in (-) to (+) direction (towards cell periphery) Voet Fig. 35-94 Kinesin Structure • Globular head: tubulin-binding site & nucleotide binding site • flexible neck linker • a-helical stalk leading into coiled-coil • ATP hydrolysis triggers conformational change in neck linker via 2 switch regions: • When ATP is bound, neck linker docks with catalytic core • Upon ATP hydrolysis, the neck linker “unzips” Voet Fig. 35-95 & 96 & 97 Kinesin Cycle Voet Fig. 35-98 Hand-over-Hand Mechanism ATP-bound state: strong microtuble binding ADP-bound state: weak microtubule binding 1. 6. ATP binds to leading head - globular kinesin head which is already bound to the microtubule and oriented towards (+) end Neck linker of leading head “zips up” agains catalytic core trailing head is thrown forward (trailing head has bound ADP and reduced affinity to microtubule): Trailing head swings by ~ 160 Å, net movement of dimeric kinesin is ~ 80 Å = length of one microtubule dimer ATP in new trailing head is hydrolyzed & phosphate released: affinity for microtuble decreases ADP in new leading head dissociates Two heads work in a coordinated fashion ATP binding to leading head induces power stroke 2. 3. 4. 5. Processivity Kinesin is highly processive: it takes several 100 steps on a microtubule without detaching or sliding backwards! How? • coordinated, but out of phase ATP cycle in both heads • one head is always firmly attached to microtubule Movie demonstrating kinesins processivity: http://www.proweb.org/kinesin/axonemeMTs.html Dynein • 1 or more heavy chains (motor domain) • several intermediate and light chains • motor domain is 7-membered ring, ATPhydrolyzing unit • coiled-coil extension forms stalk that interacts via globular domain with microtubules • long stem (with intermediate and light chains) binds cargo Valle, Cell 2003; Voet Fig. 35-107