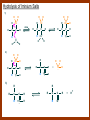* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Aldehydes Ketones
George S. Hammond wikipedia , lookup
Discodermolide wikipedia , lookup
Kinetic resolution wikipedia , lookup
Elias James Corey wikipedia , lookup
Aromatization wikipedia , lookup
Stille reaction wikipedia , lookup
Metal carbonyl wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Ene reaction wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Petasis reaction wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Aldol reaction wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Hydroformylation wikipedia , lookup
Asymmetric induction wikipedia , lookup
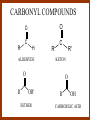
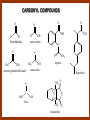
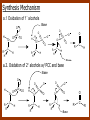
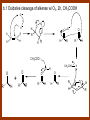
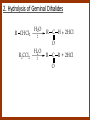
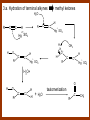
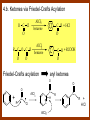
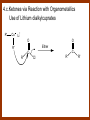
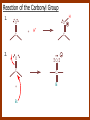
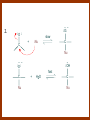
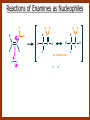
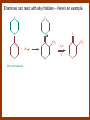
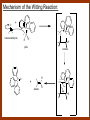
CARBONYL COMPOUNDS: NUCLEOPHILIC ADDITION REACTION Lecture 6 ORGANIC CHEMISTRY 2 CARBONYL COMPOUNDS R O O C C H ALDEHYDE R R' KETON O R O OR' ESTHER R OH CARBOXILIC ACID CARBONYL COMPOUNDS Formalin Ibuprofen Aspirin Asam cuka Asam semut Perasa buah CARBONYL COMPOUNDS O OH H OH asam semut H H Formaldehida OH O O H3C O O O O H3C CH3 Aseton (pembersih kutek) O aspirin OH asam cuka ibuprofen CH3 O C H2N NH2 Urea CH3 O O O CH3 O kantaridin Preparation of Aldehydes and Ketones 1. 2. 3. 4. Oxidation reactions Hydrolysis of geminal dihalides Hydration of alkynes Reactions with acid derivatives and nitriles 5. Reaction with carboxylic acids 6. Reaction with thioacetals 1. Aldehydes/Ketones via Oxidation Reactions a. From Alcohols via PCC b. From Alkenes via Ozonolysis c. From Glycols via Periodic Acid Cleavage RCH 2 OH PCC R C H O R 2 CHOH PCC R C R O R'CH CR 2 ozonolysis R' C O R' R C OH CH 2 OH HIO 4 H +R R C O C R O R' + H C O H Synthesis Mechanism a.1 Oxidation of 1˚ alcohols :Base O H O C R H O Cr O O O H O H Cr + O O H R C R H O O Cr O C R H O C H a.2. Oxidation of 2° alcohols w/ PCC and base H :Base :Base O O H O O C R H R Cr O H O + R C R R H R O O O O H O Cr Cr C R O O :Base C R b.1 Oxidative cleavage of alkenes w/ O3, Zn, CH3COOH O O O H R H C C O O R H R O O H H R + O O R R H CH3COO- O R O + H + H O H O O R R H H CH3COO H R R H O O O H R b.2.Ozonolysis of alkenes, if one of the unsaturated carbon atoms is disubstituted. O R R C C O R H CH3COO O O O O + O O O O R R R R O O R CH3COO- O O O + O H O + R R R R R R R R 2. Hydrolysis of Geminal Dihalides R CH Cl2 R 2CCl2 H 2O ² R H 2O ² R C H + 2 HCl O C R + 2 HCl O 3. Hydration of Alkynes a. Markovnikov Addition b. Anti-Markovnikov Addition H2O / H2SO4 R C C H HgSO4 9-BBN R C C H - H2O2 / OH R R C C H R C OH H O C C H2 C H OH H R CH3 C O H 3.a. Hydration of terminal alkynes methyl ketones H2O R C C R H C + H C + Hg SO4 2+ Hg SO4 H H O O H C R C OH2 + H + Hg SO4 H C C R + Hg SO4 H3O+ O H O C R tautomerization H C H + H2O C R CH3 4. Reactions with Acid Halides a. Aldehydes via Selective Reduction Lithium tri-tert-butoxyaluminum hydride Rosenmund reduction b. Ketones via Friedel-Crafts Acylation c. Ketones via reaction with Organometallics Gilman reagent (organocuprates) 4.a. Aldehydes from Acid Chlorides • Lithium tri-t-butoxyaluminum hydride reduction • Rosenmund reduction R C Cl O R C O Cl LiAlH(O-t-bu) 3 ether H 2 / Pd / S BaSO 4 Rosenmund catalyst R C H O R C H O 4.b. Ketones via Friedel-Crafts Acylation AlCl3 benzene R C Cl O R C O C R O O C R + HCl O AlCl3 benzene C R + RCOOH O Friedel-Crafts acylation aryl ketones O O + C AlCl3 C R Cl C + AlCl4- H O R C R + H Cl 4.c.Ketones via Reaction with Organometallics Use of Lithium dialkylcuprates R' Cu Li + O O Ether R' C C R Cl R R' 5. Aldehydes from Esters and Amides Diisobutylaluminum hydride (DIBAH or DIBAL-H) O R C OR' or O R C NH 2 or O R C NHR' or O R C NR 2 ' 1. Diisobutylaluminum hydride 2. H3 O+ O R C H 5.a. Partial reduction of certain carboxylic acid derivatives O H + O H O H O DIBAH, toluene R C R OR' R C O + H3O+ H R' C + R'OH H 6. Ketones from Carboxylic Acids Attack by Alkyl Lithium reagents RCOOH RLi RCOO - Li+ + RH RCOO - Li+ RLi R R R R R R C C O - Li+ H 2O O - Li+ OH ( H O ) 2 OH R R R C C O - Li+ O - Li+ OH OH C O R 8. Reactions with Nitriles a. Grignard Addition to give Ketones b. DIBAH Addition to give Aldehydes R CN R'MgX R C N MgX R' R CN DIBAH or DIBAL-H diisobutylaluminum hydride R C H O R H+ H 2O C R' O C N Al(i-bu)2 H + R H /H2 O 7. Ketones from Thioacetals a. Thioacetal formation from an aldehyde precursor b. Alkylation of the thioacetal intermediate using alkyl lithium reagents c. Hydrolysis of the alkylated thioacetal to give ketone product R C H R S C C 4H 9Li ( R H S S C 4H 10 ) C R CH 2 R' S S C Li+ (a thioacetal) H R'CH 2X R HgCl 2 / CH3OH / H2O ( S C BF 3 O S S HSCH 2CH 2SH HSCH 2CH 2 SH ) S C R R S C O + LiX CH 2 R' CH 2 R' Characteristic Reactions of Aldehydes and Ketones 1. Reduction reactions a. Alcohol formation b. Alkane formation 2. Oxidation reactions 3. Nucleophilic addition reactions a. Grignard additions to form alcohols b. Addition of water (hydration) to form gem-diols c. Addition of alcohols to form acetals/ketals d. Addition of HCN to form cyanohydrins e. Addition of ammonia and ammonia derivatives Reduction Reactions of Aldehydes & Ketones 1. Alcohol formation a. Hydrogenation b. Hydride reduction 2. Alkane formation a. Clemmensen reduction b. Wolff-Kishner reduction R C H O R C H O H 2 / Pt LiAlH4 ether R CH 2OH H 2O H + R C H conc. HCl R CH 3 Zn(Hg) O NH 2NH 2 R C H R CH 3 OH / H2O O R CH 2OH Oxidation of Aldehydes & Ketones 1. Conversion of aldehydes to carboxylic acids 2. Oxidation of aromatic aldehydes / ketones to benzoic acid derivatives 3. Haloform reaction of methyl carbonyls 4. Periodic acid cleavage of vicinal dials/diketones Aldehyde / Ketone Oxidations 1. R H or Ar C C Ag(NH3)2 H + (Tollens reagent) RCOOH (ArCOOH) O O C 2. H O KMnO 4 or K2Cr 2O 7 or C ² COOH R O 3. CH 3 C R - X2 OH / H2O HCX 3 + RCOO O 4. R C C O O H HIO 4 RCOOH + HCOOH + HIO 3 Nucleophilic addition reactions Structure of the Carbonyl Group C O Hybridization of the carbonyl carbon is sp2. Geometry of the carbonyl carbon is trigonal planar Attack by nucleophiles will occur with equal ease from either the top or the bottom of the carbonyl group. The carbonyl carbon is prochiral. That is, the carbonyl carbon is not the center of chirality, but it becomes chiral as the reaction proceeds. Prochiral Nu C :Nu OH R' R These two products are enantiomers. R' C O R :Nu R R' C Nu OH In general, both enantiomers are formed in equal amount. Reaction of the Carbonyl Group 1. H O C 2. O + H+ C O O C C + B B: Nucleophilic Addition to Carbonyl: General Mechanism 1. + :OH :O : C + + H C .. : OH + : OH C fast slow + :Nu C Nu 2. .. _ :O: :O : C slow + :Nu C Nu .. _ :O: .. : OH fast C Nu + H2O C Nu Relative Reactivity of Aldehydes & Ketones Aldehydes >>> ketones 1. Steric Reason • nucleophile is able to approach aldehydes more readily because it only has 1 large substituent bonded to the C=O carbon, vs. 2 in ketones. • transition state for the aldehyde rxn is therefore less crowded and has lower energy. Aldehydes Ketones 2. Electronic Reason • greater polarization of aldehyde carbonyl group • aldehyde is more electrophilic and more reactive than ketones. H R C H + H 1˚ carbocation (less stable, more reactive) O R R C + R' 2˚carbocation (more stable, less reactive) ς- ς+ + C O ς- ς+ + H Aldehyde (less stabilization of ς+, more reactive) R C R' Ketone (more stabilization of ς+, less reactive) Relative Reactivity of Aldehydes & Ketones Aliphatic aldehydes >>> Aromatic aldehydes The electon-donating resonance effect of the aromatic ring makes the carbonyl group less electrophilic than the carbonyl group of the aliphatic aldehyde. The carbocation intermediate H H O O C C The positive charge character on carbon makes this an excellent site for attack by Lewis bases (nucleophiles). Nucleophile attacks the electrophilic C=O carbon from a direction ~45˚ to the plane of the carbonyl group At the same time: Rehybridization of the carbonyl carbon from sp2 to sp3 occurs. Once we have the intermediate, what happens to it? Case 1: The Addition Product is Stable. OH R C R Nu The reaction stops here. This happens most often when the nucleophilic atom is carbon, oxygen, or sulfur. Case 2: Addition-Elimination OH R R C R R C O + H H Nu Nu H The addition product is unstable with respect to loss of a molecule of water. This is observed most often when the nucleophilic atom is nitrogen or phosphorus. Case 3: Loss of Leaving Group O O R C Nu X R C + X Nu This process is observed when X is a potential leaving group. In this case we have nucleophilic acyl substitution. Nucleophilic Addition of H2O: Hydration Nucleophilic addition of water is catalyzed by acid and base. 1. Base-catalyzed 1. O O- slow OH :OHO H 2, H OH O- fast + OHOH OH 2. Acid-catalized 1) H O O + C H+ O H C C Resonance places greater positive charge character on the carbonyl carbon. 2) O H H O slow C C O O H H H H 3) H H H O O C C O O H + H H+ Important only for low-molecular-weight aldehydes Examples: O C H in water 20 °C >99.99% hydrate H O 58% C CH3 H O ca. 0% C CH3 CH3 Nucleophilic Addition of Alcohols: Acetal Formation Acetals and Ketals are formed by reacting two equivalents of an alcohol with an aldehyde or ketone, in the presence of an acid catalyst. Hemiacetals and Hemiketals are formed by reacting only one equivalent of alcohol with the aldehyde or ketone in the presence of an acid catalyst. Further reaction with a second alcohol forms the acetal or ketal. A diol, with two –OH groups on the same molecule, can be used to form cyclic acetals. All steps in acetal/ketal formation are reversible. O + C O H + H ROH R R' C R' R O R a hemiacetal Aldehydes form hemiacetals faster than ketones O O H H R C O R' R + ROH R + R C R' + H2O O R an acetal (or ketal) This reaction is also reversible. But, in this case, the equilibrium can be driven to the right by an application of Le Châtelier’s Principle. OR R OR H R OR OR OH OH R H OR R R R OR Mechanism of Acetal Formation: H : O: :O + H H + Cl :O + .. .. aldehyde/ketone R O: H O .. .. H R H + R :O + H + OH2 Cl H H H O .. R R O Hemiacetal/ Hemiketal .. .. O H :O R + R O: H :O .. .. H O H H :O O R O R O Acetal/ Ketal R + H3O + + + H3O Example Nucleophilic Addition of Alcohols 1. Formation of 2,2-Dimethoxy-propane O CH3 C CH3 O CH3 C CH3 O CH3 dry acid + CH3 2 CH3OH 2. Formation of a Cyclic Acetal O Dry acid = HCl gas HCl in methanol HOTs + H2 O O CH 2 CH 2 dry HCl O + HO CH 2 CH 2 OH Ethylene glycol 1,2-Ethanediol a 1,3-dioxolane + H 2O 3. Cyclization of Monosaccharides Carbohydrates contain the functional groups of alcohols and aldehydes or ketones in the same molecule. They are polyhydroxyaldehydes or polyhydroxyketones. Thus they can form acetal-type products through the intramolecular interaction of these functional groups. As a model, consider the reaction: H O CH2 HO CH2 CH2 C H+ C CH2 OH H CH2 O CH2 CH2 CH2 O H H HO H H 1 1C C 2 H OH 3 HO H 4 OH H H OH .. O .. 5 6 H H 2 OH 3 :O: H 4 OH 5 6 CH2 OH CH2 OH H : O: O O H .. OH H 6 H a pyranose ring O O: H O H 5 OH a furanose ring Nucleophilic Addition of HCN 1) O O slow C R R R C R C C N N 2) O R C R + H+ R O H C R C C N N A cyanohydrin Example O O + C N Na2CO3 C N (NaCN) •Notice that the cyanide ion and the acid are added in two separate steps! •Sodium carbonate is used to keep the reaction medium basic. H2O, H+ OH C N So, what’s it good for? OH 1. LiAlH4,THF CHCH2NH2 2. H2O OH O 2-Amino-1-phenylethanol CHCN HCN OH H3O, heat CHCO2H Mandelic Acid Addition of Organometallic Reagents R R - "R: " slow + C O R R' C O R' (from R-MgX) H+ R, R' = H, alkyl, or aryl R R C R' The products of the addition are always alcohols. OH Whatever is attached to the carbonyl group will be attached to the resulting alcohol carbon. H H R M + (M = Li or MgX) C O H formaldehyde R M + R C O H other aldehydes R M + C OH H secondary alcohol R R R OH H primary alcohol R R C C R' ketones O R C OH R' tertiary alcohol Nucleophilic Addition of Grignard (R-MgX) O + MgX R MgX MgX O O R R Tetrahedral intermediate OH2 OH + R An Alcohol HOMgX Addition of Hydride Reagents O O R ":H-" R' fr:NaBH4 OH H3O C R R' C H R R' H Compounds that bear an amino group G NH 2 Form Imines The G group can be one of many different possibilities Addition-Elimination: The Formation of Imines G .. NH2 R R C + R O HA C N G + H2O R an imine All of the imine reactions, regardless of G, go by the same mechanism. Mechanism of Imine Formation: Step 1 H R .. NH2 G C + slow .. O .. G R R + N C H R .. _ O .. : G R .. N C H R Step 2 R G .. N C H R .. OH .. R fast G HA + + N C + H2 O + _ A + H R H Step 3 R G + N H _ C + R A fast G .. N R C R A .. OH .. Formation of Simple Imines A. Simple primary amines R R C R O .. + H2N R acid C N R + H2O R an imine Aldehydes and ketones react with simple primary amines to yield imines. The equilibrium is unfavorable; the products are much less stable than the reactants. B. Simple secondary amines When secondary amines are allowed to react with aldehydes or ketones, dehydration of the type shown in the elimination step cannot take place (there is no labile hydrogen on the nitrogen atom of the addition product). OH R C R N R' R' If the starting aldehyde or ketone has an α -hydrogen, however, dehydration toward the α -carbon can occur, yielding an enamine. R H O C C + H R R R2NH + R H + R OH C C R NR2 R NR2 C R H + C H2O R an enamine The acid catalyst is generally a dry acid, such as p-toluene sulfonic acid (HOTs) Amines that are used typically to form enamines: CH3 CH2 N CH3 CH2 Diethylamine H N H Piperidine O N H N Pyrrolidine H Morpholine Enamine Formation 1) R H :O : C C R + H + R R .. + OH C C R R R R 2) H H .. + OH C C R .. N R R H R slow R H .. :OH C C R R +N H R R H .. :OH C C + R R H .. +OH2 C C R N: R R R R 3) + .. R H OH2 H C C R N: R R R H + C C R N: R R R R R + H2O H + C C R N: R R R R C C R N: R R R C R N+ R 4) R C + H + R R Formation of Oximes R R C O + .. H 2N OH acid C N OH + H2O R R hydroxylamine an oxime Aldehydes and ketones react with hydroxylamine to yield oximes. Oximes are important derivatives in qualitative organic analysis. Formation of Hydrazones R R C O + .. H2 N acid C NH R N NH R + H2O R R a hydrazine a hydrazone Aldehydes and ketones react with substituted hydrazines to yield substituted hydrazones. The equilibrium is generally unfavorable. Exception: when R is an aromatic ring. Wolff-Kishner Reaction: Nu- Addition of Hydrazine N O + R N H2NNH2 R' -OH + OH2 R' R N R OH2 H OH + R H R' alkane OH2 + OH2 C N N+ R R' H R' R NH2 N H N N H OH R R' H C N R' H Formation of Semicarbazones O R C R O .. + H2N acid NH C NH2 semicarbazide O R C N NH C NH2 + H2O R a semicarbazone Aldehydes and ketones react with semicarbazide to yield semicarbazones. Semicarbazones are the second-most important of the derivatives of aldehydes and ketones. The enamine is quite nucleophilic, owing to resonance of the type: R' R N C R R' R' R C N C R R R' C R As a consequence of this resonance, the α-carbon of an enamine has a great deal of carbanion-like (nucleophilic) character. Reactions of Enamines as Nucleophiles R :N R C R R C R R R R R R +N C C R R an iminium salt R X _ + SN2 R :N C C + R R X R R Hydrolysis of Iminium Salts 1) R R R +N R R R R N: C C R +O : R R +N H C C R R :O .. H slow R C C R .. O .. H R R H R R H H 2) R R R R +N H C C R R :O .. H R R R C C R R +O .. H + R N .. H 3) R R R C C R R +O .. H R C C R :O : R + + H Enamines can react with alkyl halides -- Here’s an example. O O N N O CH3 CH3 + CH3 I H2O H+ from cyclohexanone Nucleophilic Addition of Phosphorus Ylides: The Wittig Reaction Converts an aldehyde/ketone into an alkene. A phosphorus ylide(aka phosphorane), acts as the NuYlide : A compound or intermediate with both a positive and a negative formal charge on adjacent atoms. R (C6H5)3P C R + (C6H5)3P _ R .. C R The ylide is nucleophilic, owing to the negative charge character on carbon (structure on the right). A phosphorus ylide(aka phosphorane), acts as the Nu- to attack the carbonyl carbon and yields a four-membered ring, dipolar intermediate called the betaine. The betaine decomposes spontaneously to yield an alkene and a triphenylphosphine oxide. Can produce monosubstituted, disubstituted, and trisubstituted alkenes. R3 R1 C O + (C6H5)3P C R2 R4 R2 R1 R3 C C R4 P(C6H5)3 + a betaine : O: .. _ an ylide R1 C R2 R3 + C R4 O P(C6H5)3 This is a type of condensation reaction -- we use it to “dock” to large structures together. This is another example of addition-elimination. Mechanism of the Witting Reaction: : O: + P ketone/aldehyde : + R -: ..: + R' R' ylide P O P O R betaine R' + .. :O alkene P R R' R Conjugate Nucleophilic Addition to α-β-Unsaturated Aldehydes and Ketones Direct addition (aka 1,2 addition) occurs when a nucleophile attacks the carbon in the carbonyl directly. Conjugate addition (aka 1,4 addition) occurs when the nucleophile attacks the carbonyl indirectly by attacking the second carbon away from the carbonyl group, called the beta carbon, in an unsaturated aldehyde or ketone. Conjugate addition reactions form an initial product called an enolate, which is protonated on the carbon next to the carbonyl, the alpha carbon, to give the final saturated aldehyde/ketone product. Conjugate addition can be carried out with nucleophiles such as primary amines, secondary amines, and even alkyl groups like in organocopper reactions. It is the carbonyl that activates the conjugated C=C double bond for addition which would otherwise not react. Conjugate (1,4) addition mechanism: -: ..: O : O: Nu - O : Nu Nu H H alpha,beta-unsaturated aldehyde or ketone O+ H :O: - .. Nu Enolate ion H Saturated aldehyde or ketone