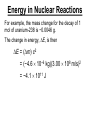* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapter #20 Nuclear Chemistry
Two-dimensional nuclear magnetic resonance spectroscopy wikipedia , lookup
Nuclear and radiation accidents and incidents wikipedia , lookup
Muon-catalyzed fusion wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Isotopic labeling wikipedia , lookup
Background radiation wikipedia , lookup
Nuclear fission product wikipedia , lookup
Nuclear fission wikipedia , lookup
Ionizing radiation wikipedia , lookup
Technetium-99m wikipedia , lookup
Nuclear fusion–fission hybrid wikipedia , lookup
Nuclear fusion wikipedia , lookup
Radioactive decay wikipedia , lookup
Nuclear binding energy wikipedia , lookup
Nuclear transmutation wikipedia , lookup
Nuclear drip line wikipedia , lookup
Valley of stability wikipedia , lookup
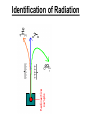
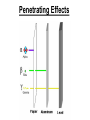
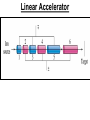
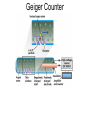
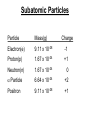
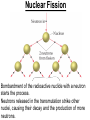
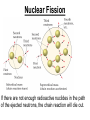
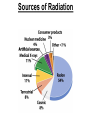
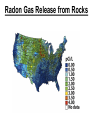
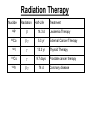
Chapter 20 Nuclear Chemistry HISTORY Radioactivity was discovered by Henry Bequerel in 1896 by observing uranium salts emit energy. Madame Curie and her husband extended Bequerel’s work on radioactivity Curie was the first to use Radioactivity to describe the spontaneous emission of alpha, beta, and gamma particles from an unstable nucleus. Both Curies suffered the effects of radiation poisoning. Rutherford took over and bombarded gold with alpha particles Identification of Radiation Penetrating Effects Nuclear Chemistry • Nuclear chemistry is the study of reactions that involve changes in the nuclei of atoms. • Radioactive decay is the spontaneous disintegration of alpha, beta, and gamma particles. • Radioactive decay follows first-order kinetics. NUCLEAR STABILITY • Kinetic stability describes the probability that a nucleus will undergo decomposition to form a different nucleus (radioactive decay) • Thermodynamic Stability - the potential energy of a particular nucleus as compared with the sum of the potential energies of its component protons and neutrons. (Binding energy) Stability radioactive decay Light elements are stable if the neutrons and protons are equal, i.e. 1:1 ration, heavier nuclides require a ratio of 1:1.5 Magic numbers of protons and neutrons seem to exist, much like 8 electrons to make elements and ions Nobel. Magic Numbers • • • • • Even number protons and neutrons are stable compared to odd ones Magic numbers (protons or neutrons) 2,8,20,28,50,82 and 126 For example tin has 10 stable isotopes, even number, but on either side of elemental tin indium and antimony have only two stable isotopes Nuclei with magic numbers of both protons and neutrons are said to be “doubly magic” and even more stable i.e. Helium-4 two protons and two neutrons and Pb-208 with 82 protons and 126 neutrons Could be shells for nucleons, like electrons Stability radioactive decay Number of stable nuclides related to numbers of protons and neurons Nuclear Stability Stability radioactive decay • • • • • Nucleons are protons and neutrons The strong nuclear force keeps the nucleus together by overcoming the repulsive force of the protons. Neutrons are present to help dissipate the repulsive forces between the protons As the atomic number (number of protons) increases, so does the number of neutrons to shield the repulsion of the protons All nuclides with 84 or more protons are unstable and radioactive. This means the strong force is only strong enough neutralize the force of 84 protons. Decay Series The HalfLives of Nuclides in the 238 U 92 Decay Series Carbon Radioactive Decay Products Name Carbon-10 Carbon-11 Carbon-12 Carbon-13 Carbon-14 Carbon-15 Carbon-16 Mass (amu) 12.00000 13.00335 Mode(s) of Natural Half-Life Decay Abundance Positron Emis. 19.45 s Positron Emis. 20.3 min (Stable) Decay Decay Decay (Stable) 5730 y 2.4 s 0.74 s 98.89 % 1.11 % Types of Radioactive Decay Decay processes Neutron-rich nuclei, converts a neutron to a proton, thus lowering the neutron/proton ration Neutron-poor nuclei, net effect of converting a proton to a neutron thus causing an increase neutron/proton ratio Heavy nuclei, Z>200 just unstable regardless of the neutron/proton ratio, just too many positive protons Decay Types Alpha particle emitters (Mass number changes) Nuclei with atomic mass number>200 The daughter nuclei contains two fewer protons and two fewer neutrons than the parent U-238, Th-230 Types of Radioactive Decay Decay processes Neutron-rich nuclei, converts a neutron to a proton, thus lowering the neutron/proton ration Neutron-poor nuclei, net effect of converting a proton to a neutron thus causing an increase neutron/proton ratio Heavy nuclei, Z>200 just unstable regardless of the neutron/proton ratio, just too many positive protons Decay Types Beta particle decay Too many neutrons Atomic number increases, thus more protons Neutron splits into a proton and electron called transmutation. 1 o 1 0n → 1 P + -1 β Examples Th-234, I-131 234 Th 90 234 91 Pa + 0 -1 e Decay Types Electron Capture Neutron-poor nuclides Electron in an inner shell reacts with a proton 1 P+ 0 β → 1 n 1 -1 0 A X Z + 0 β -1 → A X’ Z-1 + x-ray No change in mass number Example iron-55 Decay Types Positron Emission Neutron-poor nuclides Same mass as an electron, but opposite charge, the positron emission is opposite beta decay 1 P 1 A X Z → → 1 0n A X’ Z-1 Example C-11 + + 0 +1β 0 +1β Decay Types Electron Capture Neutron-poor nuclides Electron in an inner shell reacts with a proton 1 P+ 0 β → 1 n 1 -1 0 A X + 0 β → A X’ + x-ray Z -1 Z-1 No change in mass number Example iron-55 Decay Types Gamma Emission 00γ Many nuclear decay daughters are in an elevated, or excited, energy state These meta stable isotopes emit gamma rays to lower their potential energy This emission can be instantaneous, or delayed for sever hours Te-99m has a half life of about 6 hours 98 43Tc* → 98 43Tc + 0 0γ Decay Types Spontaneous Fission Very massive nuclei Z > 103 Usually large amounts of energy are released Usually neutrons are released Example: 25498Cf → 11846Pd + 13252Te + 4 10n Decay Types Various Types of Radioactive Processes Showing the Changes That Take Place in the Nuclides Radioactive Decay Radiochemical Dating • n = t/t1/2 t - time, t1/2 - time for a half-life, and n - the number of half-lives • At/Ao = 0.5n Ao - amount initially present, At - amount at time t, and n - the number of half-lives • If we know what fraction of sample is left (At/Ao) and its half-life (t1/2), we can calculate how much time has elapsed. Radiocarbon Dating of Artifacts Calibration Curves Kinetics of Radioactive Decay • Radioactive decay is a first order process, but using atoms instead of concentration Radioactive decay rates • • • Activity is defined as the number of nuclei that decay per unit time A = -ΔN/Δt, the units are usually disintegrations per second or minute (dps), dpm The activity is directly proportional to the number of atoms, thus A(Rate)=kN From Che162 we know the first order rate law is lnN/N0 = -kt Also t1/2 ln1/2N0/N0 = -kt1/2 → t1/2 = 0.693/k Example problem Fort Rock Cave in Oregon is the site where archaeologists discovered several Indian sandals, the oldest ever found in Oregon. Analysis of the 14C/12C ratio of the sandals gave an average decay rate of 5.1 dpm per gram of carbon. Carbon found in living organisms has a C-14/C-12 ratio of 1.3 X 10-12, with a decay rate of 15 dpm/g C. How long ago was the sage brush in the sandals cut? The half life of carbon-14 is 5730 years. Note dpm is disintegrations per minute Sample Problem Solution • First calculate the rate constant k from the half-life: k=0.693/5730 = 0.000121 yr-1 • Substitute into the first order rate equation. • ln(N/N0) = kt t = ln(N/N0)/k = ln(15/5.1)/0.000121 t = 8910 years old sandals Practice A mammoth tusk containing grooves made by a sharp stone edge (indicating the presence of humans or Neanderthals) was uncovered at an ancient camp site in the Ural Mountains in 2001. The 14C/12C ratio in the tusk was only 1.19% of that in modern elephant tusks. How old is the mammoth tusk? Practice Radioactive radon-222 decays with a loss of one particle. The half-life is 3.82 days. What percentage of the radon in a sealed vial would remain after 7.0 days? Nuclear Transformations • • • • • • Rutherford (1919) was the first to carry out a bombardment reaction, when he combined an alpha particle with nitrogen-14, creating oxygen-17 and a proton The next successful bombardment reaction was done 14 years later when Aluminum-27 to make phosphorus-30 and a neutron If the bombarding particle has a positive charge then repulsion by the nucleus hinders the process, thus particle accelerators are required. Cyclotron and linear accelerator pg850 Neutrons, do not suffer from the repulsive effect Synthetic elements have been made, called transuranium elements Cyclotron Nuclear reactions can be induced by accelerating a particle and colliding it with the nuclide. Cyclotron An Aerial View of Fermilab, a High Energy Particle Accelerator Cyclotron. The Accelerator Tunnel at Fermilab Linear Accelerator Linear Accelerator Cyclotron Detection and Uses of Radioactivity • • • Geiger counter, high energy from radioactive substances ionizes the Ar, thus allowing a current to flow. The more ions the more current, thus more radioactive Scintillation counter, measures the amount of light given off by a phosphor such as ZnS, which is measured by a photometer Badges Geiger Counter One can use a device like this Geiger counter to measure the amount of activity present in a radioactive sample. The ionizing radiation creates ions, which conduct a current that is detected by the instrument Geiger Counter Thermodynamic Stability • This is done by comparing the mass of the individual protons and neutrons to the mass of the nucleus itself. The difference in mass is called the mass defect (Δm), which when plugged into E = mC2, or ΔE = ΔmC2 for change in energy • The mass of an atom is always less than the mass of the subatomic particles Protium is the only exception, since there is no defect The other isotopes of hydrogen deuterium and tritium have defects Mass of neutron = 1.008665 amu Mass of proton = 1.007276 amu Mass of electron = 0.0005446623 amu , note mass of electron is not really necessary in calculations since it subtracts out when finding the difference Subatomic Particles Particle Mass(g) Charge Electron(e) 9.11 x 10-28 -1 Proton(p) 1.67 x 10-24 +1 Neutron(n) 1.67 x 10-24 0 Particle 6.64 x 10-24 +2 Positron 9.11 x 10-28 +1 Thermodynamic Stability • • • • • Just like a molecule is more stable that its atoms, an nucleus in more stable than its individual atoms. Energy changes for nuclear process are extremely large when compared to normal chemical and physical changes, thus very valuable energy source. Normal units are expressed per nucleon, in MeV (million electron volts) MeV = 1.60 X 10-13 J OR amu = 931 MeV All nuclei have different relative stabilities, see figure 18.9 Sample problem: • Calcualte the changes in mass (in amu) and energy (in J/mol and eV/atom) that accompany the radioactive decay of 238U to 234Th and an alpha particle. The alpha particle absorbs two electrons from the surrounding matter to form a helium atom. Solution (Note: AMU = g/mole) Δm = mass prod. – mass react. Δm = (mass 234Th + mass 42He) - mass238U Δm = (234.43601 + 4.002603) - 238.050788 = -0.004584 amu or -4.584X10-6kg ΔE = mC2 ↔ ΔE =( -4.584X10-6kg)(2.998X108m/s)2 =-4.120X1011 j/mole ΔE = -0.004584 amu X 931 MeV/amu Divide by the mass number to get energy per nucleon, called binding energy Practice What is the binding energy of 60Ni? The mass of a 60Ni atom is 59.9308 amu. The mass of an electron is 9.10939 x 10-31 kg and 1 amu is 1.66054 x 10-27 kg. Thermodynamic Stability Revisiting the graph on page 988 Notice that Iron is the most stable nuclide ∆E is negative when a process goes from a less stable to a more stable state In nuclear reactions more stable nuclei can be achieved by combining nuclei (fusion) or splitting a nucleus (fission) Lighter elements typically undergo fusion, while elements heavier than iron undergo fission. Thermodynamic Stability • For lighter elements, fusion processes lead to nuclei with greater binding energy, whereas heavy elements are formed through other processes. Artificial Elements • Scientists have been transmuting elements since 1919 when oxygen-17 and hydrogen-1 were produced from nitrogen-14 and particles. 14 7N + 4 2He 17 8O + 1 1H • Artificial transmutation requires bombardment with high velocity particles. • Alpha particles are positivly charged so how do they strike the nucleus, since the nucleus is positivly charged? Energy in Nuclear Reactions • In the types of chemical reactions we have encountered previously, the amount of mass converted to energy has been minimal. • However, these energies are many thousands of times greater in nuclear reactions. Energy in Nuclear Reactions For example, the mass change for the decay of 1 mol of uranium-238 is −0.0046 g. The change in energy, E, is then E = (m) c2 E = (−4.6 10−6 kg)(3.00 108 m/s)2 E = −4.1 1011 J Fission Process • Discovered in the 1930’s when U-235 was bombarded with neutrons Neutrons, due to their neutral charge do not require accelerators • • 11n + 23592U → 14156Ba + 9236Kr + 3 11n This process delivers 2.1X1013J/mole, compared to 8.0X105j of energy for the combustion of methane About 26 million times more energy Another splitting process produces the elements Te-137 and Zr-97, with two neutrons There are 200 different isotopes of 35 different element produced, thus the nucleus fragments in many different ways Fission Process • Since neutrons are produced, then it is possible to have a self-sustaining reaction If the average production of neutrons is less than one, the reaction is called subcritical If the neutron production is equal to one then it is called critical If the neutron production is greater than one then the reaction is called super-critical To achieve the a critical state, then a critical mass is required If the mass is too small then the neutrons escape before splitting other nuclei Nuclear Fission • How does one tap all that energy? • Nuclear fission is the type of reaction carried out in nuclear reactors. Nuclear Fission Bombardment of the radioactive nuclide with a neutron starts the process. Neutrons released in the transmutation strike other nuclei, causing their decay and the production of more neutrons. Nuclear Fission If there are not enough radioactive nuclides in the path of the ejected neutrons, the chain reaction will die out. Nuclear Fission Therefore, there must be a certain minimum amount of fissionable material present for the chain reaction to be sustained: critical mass. Nuclear Reactors • In nuclear reactors the heat generated by the reaction is used to produce steam that turns a turbine connected to a generator Nuclear Reactors The reaction is kept in check by the use of control rods. These block the paths of some neutrons, keeping the system from reaching a dangerous supercritical mass. Fusion Process • Combining of nuclei, such as the reaction the occurs on the sun • Problem is that the nuclei are positive in charge, thus high temperatures (4X 107K) necessary to give the nuclei the correct amount of kinetic energy to overcome the repulsion Electric current heat Laser heat • Because to the high temperature then what about containment? Nuclear Fusion Fusion would be a superior method of generating power. The good news is that the products of the reaction are not radioactive. The bad news is that in order to achieve fusion, the material must be in the plasma state at several million kelvins Hydrogen Fusion • Heavier elements formed through the process of fusion. 1 1 H H H + 1 1 H + 2 1 1 1 3 2 2 He 2 1 0 1 + e (positron) H He 3 2 4 2 He + 2 H 1 1 Effects of Radiation • • • • What happens when one is exposed to radiation? Somatic damage is damage to the organism itself Genetic damage is damage to the genetic machinery, RNA DNA for example Damage depends on the following factors Quantities of Radiation Unit Parameter Description Curie (Ci) Level of Radioactivity 3.7x1010 nuclear disintegrations/s Becquerel (B)* Level of Radioactivity 1 disintegration/s Gray (Gy) Ionizing Energy Absorbed 1 Gy = 1 J/kg of tissue mass Sievert (Sv) Amount of Tissue Damage 1Sv = 1Gy x RBE** *SI unit of radioactivity **Relative Biological Effectiveness Damage Factors • The energy of the radiation, measured in rads ( radiation absorbed dose), where one rad = 10-2 J of energy deposited per kg of tissue • Since different radioactive particles do different kinds of damage the rad is not the best way to consider the effects Penetrating ability of the radiation Gamma highly penetrating, since electromagnetic energy consisting of photons Beta particles penetrate up to one cm Alpha particles are stopped by the skin Damage Factors • Ionizing ability of the radiation Gamma radiation only occasionally ionize Alpha particles, highly ionizing and leave a trail of damage, since it is an ion itself, it will strip electrons from other substances • Chemical properties of the radiation source. Inert nuclides such as the noble gases pass through the body A radioactive substance such as iodine, can be concentrated in a specific location of the body. For iodine it is the thyroid. rem = rads X RBE About REM • • • rem is the radiation equivalent in man rbe is the relative effectiveness of the radiation in causing biologic damage, which is one for betta and gamma, and 20 for alpha Alpha particles have a higher rbe than beta and gamma, since the helium nuclei is much larger. Acute Effects of Single Whole-Body Doses of Ionizing Radiation Dose(REM) 0.05-0.25 0.25-1.0 1.0-2.0 2.0-4.0 Toxic Effect No acute effect, possible carcinogenic or mutagenic damage to DNA Temporary reduction in white blood cell count Radiation sickness: fatigue, vomiting, diarrhea, impaired immune system Severe radiation sickness: intestinal bleeding, bone marrow destruction 4.0-10.0 Death, usually through infection, within weeks >10.0 Death within hours Typical Radiation Exposures for a Person Living in the United States (1 millirem = 10-3 rem) Sources of Radiation Biological Effect of Radiation Radon Gas Release from Rocks Radiation Therapy Nuclide Radiation Half-Life Treatment 32P 14.3 d Leukemia Therapy 60Co 5.3 yr External Cancer Therapy 123I 13.3 yr Thyroid Therapy 131Cs 9.7 days 192Ir 74 d Prostate cancer therapy Coronary disease Medical Imaging Radionuclides Nuclide Radiation Emitted Half-Life (hr) Use 99mTc 6.0 Bones, Circulatory system, Various Organs 67Ga 78 Tumors in the Brain and Other Organs 201Tl 73 Coronary Arteries, Heart Muscle 123I 13.3 Thyroxin Production in Thyroid Gland Synthesis of the elements in stars • • • • • Stars are formed from the gravitational attraction of interstellar dust, mostly hydrogen The density gradually increases reaching a density of about 100g/mL, with a temperature of about 1.5X107 K At this point hydrogen begins to fuse into He-4, releasing energy, like our sun The overall reaction is 4 protium atoms combining to make helium and 2 beta particles plus 2 photons of gamma radiation The helium then concentrates in the core of the star, thus increasing the density and temperature, thus becoming a red giant star Synthesis of the elements in stars • At a temperature of about 2X108 K, the helium nuclei begin to fuse producing Be-8 • Be-8 is unstable due to low neutron numbers, and absorbs alpha particles creating C, O, Ne, Mg • The next stage is formation of a red supergiant star, where Na, Si, S, Ar, and Ca are produced • Next in the progressions is the formation of a massive red supergiant star, where Fe and Ni are formed by proton-neutron exchange reactions Finally the supernova is produced where elements with Z>28 being formed by multiple neutron captures Red Giant Supernova ChemTour: Half-Life Click to launch animation PC | Mac Students develop and test their understanding of the concepts of half-life and carbon dating by manipulating interactive graphs and working Practice Exercises. ChemTour: Fusion of Hydrogen Click to launch animation PC | Mac This ChemTour demonstrates the process by which hydrogen nuclei fuse to form helium nuclei. This nuclear reaction fuels the sun and stars and is the first step in the synthesis of heavier elements. ChemTour: Modes of Radioactive Decay Click to launch animation PC | Mac This ChemTour presents animated explanations of alpha decay, beta decay, positron emission, and electron capture. ChemTour: Balancing Nuclear Reactions Click to launch animation PC | Mac This quantitative exercise teaches nuclear equation balancing through worked examples and Practice Exercises. ChemTour: Half-Life Click to launch animation PC | Mac Students develop and test their understanding of the concepts of half-life and carbon dating by manipulating interactive graphs and working Practice Exercises. ChemTour: Fusion of Hydrogen Click to launch animation PC | Mac This ChemTour demonstrates the process by which hydrogen nuclei fuse to form helium nuclei. This nuclear reaction fuels the sun and stars and is the first step in the synthesis of heavier elements. ChemTour: Modes of Radioactive Decay Click to launch animation PC | Mac This ChemTour presents animated explanations of alpha decay, beta decay, positron emission, and electron capture. ChemTour: Balancing Nuclear Reactions Click to launch animation PC | Mac This quantitative exercise teaches nuclear equation balancing through worked examples and Practice Exercises. End Chapter #20 Nuclear Chemistry