* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Functional expression of P2 receptors in the inner ear of chicken
Cellular differentiation wikipedia , lookup
Chemical synapse wikipedia , lookup
NMDA receptor wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Killer-cell immunoglobulin-like receptor wikipedia , lookup
Signal transduction wikipedia , lookup
Leukotriene B4 receptor 2 wikipedia , lookup
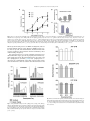
Neuroscience Letters 553 (2013) 24–28 Contents lists available at ScienceDirect Neuroscience Letters journal homepage: www.elsevier.com/locate/neulet Functional expression of P2 receptors in the inner ear of chicken embryo Fabian Galindo a , Eduardo Monjaraz a , Salvador Galicia b , Jorge Cebada b , Celso Cortés c , Amira Flores a,∗ a Instituto de Fisiología, Benemérita Universidad Autónoma de Puebla, Av. 14 Sur 6301 Colonia Jardines de San Manuel CP 72570, Puebla, Pue., Mexico Escuela de Biología, BUAP, Blvd. Valsequillo y Av. San Claudio, Edif. 112-A C.U. Colonia Jardines de San Manuel CP 72570, Puebla, Pue., Mexico c Facultad de Medicina, BUAP, Av. 13 Sur 2702 Colonia Volcanes CP 72410, Puebla, Pue., Mexico b h i g h l i g h t s • • • • ATP modulate the spontaneous afferent discharge in the inner ear during development. P2X receptors have a significative effect on the vestibular afferent discharge. P2Y receptors predominate in the basilar papilla of chicken. The purinergic effects in both systems are more important in younger animals. a r t i c l e i n f o Article history: Received 9 January 2013 Received in revised form 18 June 2013 Accepted 5 August 2013 Keywords: ATP Inner ear Development Chicken P2X receptor P2Y receptor a b s t r a c t The purpose of the present study was to investigate the modulation of spontaneous afferent activity by ATP during embryonic development in a preparation isolated chicken inner ear. This work was performed using multiunit and single-unit extracellular recordings from the posterior semicircular canal nerve and the basilar papilla nerve. ␣,-meATP, a P2X receptor agonist, notably increased the discharge frequency of the vestibular afferents between E15 and E18, but not in the basilar papilla. In contrast, the P2Y receptor agonist UTP produced a slight increase in the discharge frequency of basilar papilla afferents, without apparent changes in the vestibular afferent activity. 2-MeSATP, a P2Y agonist, increased the basal discharge of the primary afferents in a dose-age dependent way, but when we applied the antagonist of P2Y receptor, Reactive Blue 2 (10−4 M), the effect of 2-MeSATP decreased significantly. This was observed both in vestibule and basilar papilla. Using RT-PCR the presence of P2X3 , P2Y1 , P2Y2 and P2Y6 mRNA was documented in the vestibular system with more important presence during the early stage (E15) than the later stage (E21), however in the basilar papilla we found only the P2Y1 , P2Y2 and P2Y6 mRNA with the same temporal course as in the vestibule. These results confirm our pharmacological findings. Together this data suggests a role for P2X receptors-mediated purinergic signaling in vestibular synaptic organization. Temporal changes in P2Y receptors during development might be involved in the establishment of the endolymphatic ion composition needed for normal vestibular and auditory transduction and/or specific cellular differentiation. © 2013 Elsevier Ireland Ltd. All rights reserved. 1. Introduction Extracellular nucleotides, particularly adenosine 5 triphosphate (ATP), are important signaling molecules that mediate diverse biological effects via cell surface receptors [1,4]. ∗ Corresponding author at: Instituto de Fisiología, Laboratorio de Neurobiología, Benemérita Universidad Autónoma de Puebla, Apartado Postal 406, Puebla, Pue., CP 72000, Mexico. Tel.: +52 222 2 29 55 00x7303; fax: +52 222 2 29 55 00x7323. E-mail addresses: [email protected], amira.fl[email protected] (A. Flores). 0304-3940/$ – see front matter © 2013 Elsevier Ireland Ltd. All rights reserved. http://dx.doi.org/10.1016/j.neulet.2013.08.010 ATP can act at both ionotropic (P2X) and metabotropic receptors (P2Y). Multiple isoforms of P2X and P2Y receptors, which differ in molecular structure, kinetics and sensitivity for agonists and antagonists have been cloned and characterized [4,10,15]. Important developmental roles including cell differentiation, neurogenesis, gastrulation, survival and maturation of neurons, have been suggested for these receptors [6]. Although ATP and their receptors have been localized in the inner ear [18], little is known concerning the potential role of ATP in the regulation of the hair cells response, neurotransmitter release, discharge of the afferent neurons and their participation in the process of maturation during inner ear development. The present study F. Galindo et al. / Neuroscience Letters 553 (2013) 24–28 25 Table 1 Primer pairs used in the RT–PCR reactions. Target mRNA P2X1 P2X3 P2Y1 P2Y2 P2Y6 actin GenBank access no. NM204519 XM426413 NM205333 XM425667 NM205195 NM 001017992 Upper and lower primer sequences 5 -CAGACACAGGACAGCATCG-3 5 -GTCACAGTTCCAGTCGATG-3 5 -GACACTGTCATCGAGTCCT-3 5 -GGACGCTGTTCTTGATGAAG-3 5 -CGTCATGTGCAAGCTGCAG-3 5 -TCAGAGCTTTCACGATCAGC-3 5 -CACCACGTACATGTTCAACC-3 5 -CATCACCGAACTGTATATGAC-3 5 -CGTTGTCATTGGGCAGATCT-3 5 -CAATCAGCTCGTCTTTCTGG-3 5 -CCTGCTTGCTGATCCACATCTGCTG-3 5 -ATCCTGCGTCTGGACCTGGCTG-3 was performed to examine a potential role for ATP in the excitability of the avian inner ear. For this purpose, we investigated the effects of P2X and P2Y agonists and antagonists in the firing rate of the afferent fibers from the posterior semicircular canal and from the basilar papilla of the chicken during embryonic development. In addition, we studied the temporal course of P2X and P2Y receptor expression in the vestibular periphery and the basilar papilla. 2. Materials and methods 2.1. Embryo preparations All procedures described in this study were in accordance with the technical guidelines for production, care, and use of animals in the laboratory issued by SAGARPA México (NOM-062 ZOO-1999) and by the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize animal suffering and to reduce the number of animals used. We used chicken embryos (E) (Gallus domesticus) of 15, 18 and 21 days supplied by ALPES (Tehuacán, Mexico). The age of the embryos given in days was established by reference to the staging criteria of Hamburger and Hamilton (1951) [8]. Embryos were cooled, removed from the egg, and decapitated. The inner ear was dissected out in Ringer’s solution containing (in mM): NaCl 124; KCl 5; NaH2 PO4 2.2; CaCl2 2; NaHCO3 26; MgSO4 2 and glucose 10; with a pH of 7.3–7.4 after saturation with 95% O2 and 5% CO2 [25]. The isolated preparation (vestibular end organs or basilar papilla) was transferred to a recording chamber with a constant temperature of 37 ◦ C (TC-102 of Medical System Co). 2.2. Electrophysiological recording Multiunit extracellular recordings were obtained from the posterior semicircular canal nerve using a suction electrode. Electrical activity amplified by means of a conventional AC amplifier (Model P511, GRASS) was fed to an oscilloscope (Tektronix 2201) and to a window discriminator (121 WPI), the output of which was passed to a microcomputer for on-line analysis of the neuronal spike discharge rate [27]. In the basilar papilla, extracellular single-unit recordings were made with glass micropipettes filled with 2 M NaCl (30–50 M). Signals were digitized and sampled at 100 kHz (Digidata 1320A; Axon Instruments). The analyses of the spikes were made with the pClamp 9.0 (Molecular Devices) software. 2.3. Drug application All chemicals were purchased from Sigma–Aldrich. Drugs were either bath substitution – or locally applied as indicated. Data are presented as mean ± SEM, and statistical analysis performed using Annealing temperature (◦ C) RT-PCR product (bp) 58 621 58 384 64 370 58 402 60 544 65 578 Student’s paired or unpaired t test and analysis of variance (ANOVA) followed by post hoc Newman–Keuls test. 2.4. Standard RT-PCR Total RNA from crista ampullaris and basilar papilla was isolated using Quick-RNATM MiniPrep (Zymo Research, USA) according to the manufacturer’s directions. The concentration of total RNA for each sample was determined by absorbance measurements at 260 and 280 nm (Biophotometer, Eppendorf, USA.). The integrity of the RNA was assessed by electrophoresis on a denaturing agarose gel. RNA samples were stored at −30 ◦ C until analyzed by RT–PCR. cDNA was synthesized using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, USA). cDNA obtained was subjected to the PCR protocol as follows: each reaction mixture (20 l final volume) included 10 l PyroStartTM Fast PCR Master Mix (Fermentas, USA), 1 l (1 g/l) of each specific primer (Table 1), 6.5 l of water and 1.5 l (50 ng/l) of cDNA and reaction mixtures were processed in a Mastercycler gradient (Eppendorf, USA). For amplification an initial denaturation step at 94 ◦ C for 5 min was followed by 35 cycles of PCR. Each cycle consisted of three temperature steps: 30 s at 94 ◦ C, 30 s at the optimal annealing temperature (Table 1), and 60 s at 72 ◦ C. Amplification was concluded with a final elongation step at 72 ◦ C for 10 min. To verify their identity, amplification products were resolved by electrophoresis in 1.5% agarose gels and visualized by ethidium bromide staining, and the intensities were then measured by scanning the gel with the ChemiDoc Gel Documentation System (Bio-Rad, USA). Controls without reverse transcriptase were used to demonstrate the absence of contaminating DNA. PCR products were sequenced and were cleaned using QIAquick PCR purification columns (Qiagen; USA). The suitability of the gelpurified PCR product was evaluated by automatic DNA sequencing using an ABI PRISM 310 sequence analyser (Perkin–Elmer Applied Biosystems, USA). 3. Results 3.1. Vestibule We studied the effect of ATP, ␣-meATP, UTP and 2MeSATP on the basal discharge of the vestibular afferents. Fig. 1A, shows that ATP (10−8 –10−4 M) significantly enhanced the spontaneous afferent electrical activity in a dose-age dependent manner. The maximum effect was observed in E15 and E18. Likewise, application of the P2X receptor agonist ␣-meATP (10−9 –10−4 M), showed an important excitatory effect and increased the activity in a doseage dependent manner. 10−4 M caused the main excitatory effect in E15 (208 ± 4.4%), E18 (226 ± 6.7%), and E21 (197 ± 4.1%) compared with basal discharge (Fig. 1B). UTP, a P2Y agonist, (10−6 –10−3 M) did not change the electrical activity at any age studied (not shown). However, the P2Y agonist 26 F. Galindo et al. / Neuroscience Letters 553 (2013) 24–28 with age. The P2X3 receptor had a particular behavior (Fig. 3A); its expression was different at the three ages, showing increased expression at E18 over E15 and E21 (187% and 167%, respectively). This results match with the electrophysiological findings where the P2X agonist ␣,-meATP had a major effect at age E18. Expression of P2X3 , P2Y1 and P2Y2 receptors was also dependent on age; reaching its maximum levels at E21 (Fig. 3B and C). P2Y6 had an important expression, as P2Y2 , but there were not statistical differences between the ages tested (Fig. 3D). 3.2. Basilar papilla We explored the effect of ATP receptors activation on auditory afferents discharge. Fig. 4A shows that ATP 10−3 M increased the basal discharge at the three tested ages (E15, 25.73 ± 3.83%; E18, 30.57 ± 1.8% and E21, 7.77 ± 1.7%). We did not find significant changes with ␣,-meATP (10−5 –10−3 M) (not shown), but when UTP and 2-MeSATP were applied (10−3 M) we observed an increase in discharge frequency of the afferents at E15 and E18, (Fig. 4B and C). Application of Reactive Blue 2 (100 M) by bath substitution decreased significantly the spontaneous activity (>50%), and the excitatory effect of 2-MeSATP was completely abolished at all ages (not shown). Interestingly, the RT-PCR assays showed that the basilar papilla only expressed mRNA encoding for P2Y receptor isoforms. P2Y2 receptor showed a significant increase in its level of expression with increased age of the chick embryo (Fig. 3C). 4. Discussion Fig. 1. ATP and ␣-meATP effects on the basal discharge of vestibular afferents at different developmental ages. (A) ATP dose-response-age curve fitting with the Hill equation yielded, (E15) EC50 = 4.031 × 10−7 (pEC50 6.395 ± 0.3599); Hill coefficient (nH) = 0.65 ± 0.223); (E18) EC50 = 4.42 × 10−6 (pEC50 5.354 ± 0.05978); nH = 0.87 ± 0.0714; (E21) EC50 = 3.134 × 10−6 (pEC50 5.504 ± 0.4259) nH = 0.513 ± 0.333. The effect reached its maximum level at the highest concentrations tested at E18 (181.0 ± 2.157%). (B) ␣-meATP doseresponse-age curve fitting. (E15) EC50 = 3.53 × 10−6 (pEC50 5.451 ± 0.2596) nH = 0.64 ± 0.29; (E18) EC50 = 9.66 × 10−6 (pEC50 5.015 ± 0.030), nH = 0.72 ± 0.31; (E21) EC50 = 6.029 × 10−6 (pEC50 5.220 ± 0.092), nH = 0.59 ± 0.06. The maximal responses for E15 (205.6 ± 11.7%) and E18 (234.7 ± 21.21%) were always greater than the changes produced in E21 (185 ± 3.804%). Points are the means ± SEM and the line drawn is the best-fit curve obtained. 2-MeSATP, applied by microperfusion (10−5 –10−3 M), increased the spontaneous electrical activity of the primary afferents in doseage dependent manner. The effect was more apparent in E15 than in E21 (192.4 ± 18.9% vs 132.87 ± 6.81%) with 10−3 M (Fig. 2A). When we applied the antagonist of P2Y receptors, Reactive Blue 2 (10−4 M), a decrease in afferent spontaneous activity was observed at the three ages tested (Fig. 2B). Likewise, the stimulatory effect of 2-MeSATP (10−3 M) on the basal discharge in E15 is reduced to 79.6 ± 1.98% in the presence of Reactive Blue 2, 10−4 M (Fig. 2C). Using RT-PCR we detected the presence of P2X3 , P2Y1 , P2Y2 and P2Y6 receptors, however, their pattern of expression differed P2 receptors have been found in different structures of the mammalian inner ear [2–4,9,16,17,22,26,28]; however this is the first study where the presence and possible functional role of P2 receptors in the avian inner ear are demonstrated during embryonic development. Spontaneous activity in the auditory nerve fibers before the onset of hearing is essential for the survival of target neurons in the cochlear nuclei, accurate wiring of auditory pathways, and the refinement of the tonotopic maps [14,20,30]. Tritsch et al. [29] demonstrated that supporting cells within Kolliker’s organ in the rat cochlea initiates bursts of electrical activity in spiral ganglion neurons before the onset of hearing through ATP-dependent excitation of hair cells. These results suggest that peripheral, non-sensory cells are essential for the maturation of auditory pathways in the brain. Recent results show that spontaneous impulses in inner hair cells are intrinsically generated throughout pre-hearing stage of development and does not require waves of depolarization linked to ATP release [11]. This intrinsic activity disappears at the onset of hearing due to a change in the ion channels expressed by the hair cells [12]. However, Jones et al. [13] showed that in the chicken embryo the E12–E16-17 period of endogenous cochlear signaling is critical for neurotrophic support of central relay nuclei for guiding normal developmental refinements in central binaural processing pathways. After this period, the cochlear sensor acquires the ability to perform ambient sound detection and encoding. In our study, design of the oligonucleotides for the molecular identification of P2X and P2Y receptor subunits was made in concordance with the results of the pharmacology used for electrophysiological recordings of the basal discharge from the afferent neurons and according to the results obtained by other authors (3, 5, 9, 21, 22). In the vestibular system of E15 and E18 we found an important excitatory effect evoked with the P2X agonist ␣,-meATP. The RT- F. Galindo et al. / Neuroscience Letters 553 (2013) 24–28 27 Fig. 2. Effect of a P2Y agonist (2MeSATP) and a P2Y antagonist (Reactive Blue 2) on the basal discharge of vestibular afferents at different developmental ages. (A) 2MeSATP dose-response-age curve fitting with the Hill equation yielded (E15) EC50 = 2.86 × 10−6 (pEC50 = 5.543 ± 0.1354); nH = 0.83 ± 0.09; (E18) EC50 = 161.8 × 10−6 (pEC50 = 3.791 ± 0.0789); nH = 1.03 ± 0.32; (E21) EC50 = 18.0 × 10−6 (pEC50 = 4.744 ± 0.08101); nH = 1.23 ± 0.23.The maximal responses for E15 (188.4 ± 3.301%) and E18 (182.9 ± 3.872%), were greater significantly than for E21 (134.2 ± 1.131%). On mature embryos (E18 and E21) only high concentrations of 2MeSATP (10−3 M) induced an increase in basal discharge. Points are the means ± SEM and the line drawn is the best-fit curve obtained. (B) The effect of Reactive Blue 2 (10−4 M) is clearly observed at all embryonic ages but particularly at E15 (*, p < 0.05). (C) Reactive Blue 2 (10−4 M) blockage of the excitatory effect of 2MeSATP (10−3 M; *, p < 0.05). PCR assays showed the presence of mRNA encoding P2X3 , but not for P2X1 and P2X2 (data no show). However, mRNA for P2Y receptors was also present. Consonant with this, incubation with a P2X receptors agonist by microperfusion showed a stimulatory effect on the discharge frequency. The synaptogenesis process in the vestibular system has been divided into three stages (presents at periphery and central level): early synaptogenesis (E8–E9), mid-synaptogenesis (E11–E13), and late synaptogenesis (E14–E17) [7,24]. Late synaptogenesis is critical for the establishment of the final contacts between hair cells and afferents as well as between the afferent neurons and Fig. 3. Semi-quantitative analysis of P2X3 (384 bp), P2Y1 (370 bp), P2Y2 (402 bp) and P2Y6 (544 bp) mRNA expression in the vestibular end organs and basilar papilla of chick embryos at E15, E18 and E21. Each panel shows the amplified mRNA for the corresponding receptor, bar graphs show the values of band intensity (arbitrary units) normalized with respect to -actin. Values are expressed as mean ± S.E.M. (n = 4; * p < 0.05). Fig. 4. Effect of ATP and P2Y agonists (2MeSATP and UTP) on the basal discharge of auditory afferents at different developmental ages. ATP and both agonists induce (at 10−3 M concentration) an increase of the electrical activity at E15 and E18 (*, p < 0.05; **, p < 0.01). All drugs were tested at lower concentrations but no significant effects were observed. 28 F. Galindo et al. / Neuroscience Letters 553 (2013) 24–28 the second order neurons into the vestibular nucleus, at central level. Possibly ATP is helping neurons to find and establish the correct synapses at periphery and central level in the vestibular system. Consistent with this, our results in E21 showed minimal changes, probably because the synaptic contacts are established and the ATP role in this context may not be needed. Similar findings were observed in the auditory system, where the youngest animals responded better to the action of ATP. In the basilar papilla, the most prominent increase in electrical activity evoked by ATP and the P2X receptor and P2Y receptor agonists was observed at E15, an important moment for the establishment of hearing function, and we did not observe any important change in E18 and E21, periods where the hearing has started already. Regarding new possible roles for P2Y receptors, it is worth mentioning that in supporting cells and epithelial cells some experiments indicate that ATP, acting through a highly sensitive purinergic/IP3 -mediated signaling pathway, is the principal paracrine mediator implicated in the propagation of Ca2+ waves [23]. Cells of the outer sulcus participate in the absorption of cations and it is possible that these cells are responsible for maintaining the correct concentration of K+ ions [19]. The P2Y4 receptors are expressed in developing outer sulcus cells, and then down-regulated in the adult [26]. Homeostasis of the luminal fluid composition is also controlled by P2Y receptors in the apical membrane of strial marginal cells and vestibular dark cells of rodents [20,22]. Moreover, P2Y1 receptor expression has been demonstrated in several structures during chicken embryonic development [5,21]. The present study revealed for the first time by RT-PCR the presence of P2X3 , P2Y1 , P2Y2 and P2Y6 – mRNA in the inner ear of chicken embryos in three ages (E15, E18 and E21), with a temporal variation in their expression patterns. Our results suggest that ATP could to modulate the spontaneous activity of the auditory and vestibular afferent fibers at different embryonic developmental stages in the chicken inner ear. The P2X receptors in the vestibule have a very important effect on the afferent discharge while P2Y receptors maintain a more discreet profile. In the basilar papilla, only found the presence of P2Y receptors. In both organs, the purinergic effects are more important in younger animals probably because during defined moments in the embryonic development, ATP is able to help to establish synaptic contacts or to maintain an early communication between hair cells and primary afferents or may play an important role in signal transduction in the inner ear. Later, ATP may be substituted by other substances like glutamate that probably persist during the adult stage. Acknowledgments We thank Dr. Ricardo Félix for critical reading of the manuscript, and Dr. Antonio Arias-Montaño for his help with data analysis. This work was partly supported by PIFI-SEP 2012 and VIEP-BUAP SAL/G/2013 grants to A.F. References [1] M.P. Abbracchio, G. Burnstock, A. Verkhratsky, H. Zimmermann, Purinergic signalling in the nervous system: an overview, Trends Neurosci. 32 (2009) 19–29. [2] A. Aubert, C.H. Norris, P.S. Guth, Influence of ATP and ATP agonists on the physiology of the isolated semicircular canal of the frog (Rana pipiens), Neuroscience 62 (1994) 963–974. [3] U. Brändle, H.P. Zenner, J.P. Ruppersberg, Gene expression of P2X-receptors in the developing inner ear of the rat, Neurosci. Lett. 273 (1999) 105–108. [4] G. Burnstock, Physiology and pathophysiology of purinergic neurotransmission, Physiol. Rev. 87 (2007) 659–797. [5] R.C. Choi, M.L. Man, K.K. Ling, N.Y. Ip, J. Simon, E.A. Barnard, K.W. Tsim, Expression of the P2Y1 nucleotide receptor in chick muscle: its functional role in the regulation of acetylcholinesterase and acetylcholine receptor, J. Neurosci. 21 (2001) 9224–9234. [6] N. Dale, Dynamic ATP signalling and neural development, J. Physiol. 586 (2008) 2429–2436. [7] D.J. Fink, D.K. Morest, Formation of synaptic endings by colossal fibers in the vestibular epithelium of the chick embryo, Neuroscience 2 (1977) 229–252. [8] V. Hamburger, H.L. Hamilton, A series of normal stages in the development of chick embryo, J. Morphol. 88 (1951) 49–92. [9] K. Ito, Y. Chihara, S. Iwasaki, Y. Komuta, M. Sugasawa, Y. Sahara, Functional ligand-gated purinergic receptors (P2X) in rat vestibular ganglion neurons, Hear. Res. 267 (2010) 89–95. [10] M.F. Jarvis, B.S. Khakh, ATP-gated P2X cation-channels, Neuropharmacology 56 (2009) 208–215. [11] S.L. Johnson, H.J. Kennedy, M.C. Holley, R. Fettiplace, W. Marcotti, The resting transducer current drives spontaneous activity in prehearing mammalian cochlear inner hair cells, J. Neurosci. 32 (2012) 10479–10483. [12] S.L. Johnson, T. Eckrich, S. Kuhn, V. Zampini, C. Franz, K.M. Ranatunga, T.P. Roberts, S. Masetto, M. Knipper, C.J. Kros, W. Marcotti, Position-dependent patterning of spontaneous action potentials in immature cochlear inner hair cells, Nat. Neurosci. 14 (2011) 711–718. [13] T.A. Jones, S.M. Jones, K.C. Paggett, Emergence of hearing in the chicken embryo, J. Neurophysiol. 96 (2006) 128–141. [14] K. Kandler, Activity-dependent organization of inhibitory circuits: lessons from the auditory system, Curr. Opin. Neurobiol. 14 (2004) 96–104. [15] E.R. Lazarowski, R.C. Boucher, T.K. Harden, Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules, Mol. Pharmacol. 64 (2003) 785–795. [16] J.H. Lee, J.H. Heo, S.O. Chang, C.S. Kim, S.H. Oh, Reactive blue 2, an antagonist of rat P2Y4 , increases K+ secretion in rat cochlea strial marginal cells, Hear. Res. 219 (2006) 66–73. [17] J.H. Lee, J.H. Heo, C.H. Kim, S.O. Chang, C.S. Kim, S.H. Oh, Changes in P2Y4 receptor expression in rat cochlear outer sulcus cells during development, Hear. Res. 228 (2007) 201–211. [18] F. Mammano, ATP-dependent intercellular Ca2+ signaling in the developing cochlea: facts, fantasies and perspectives, Semin. Cell Dev. Biol. 24 (2013) 31–39. [19] D.C. Marcus, T. Chiba, K+ and Na+ absortion by outer sulcus epithelial cells, Hear. Res. 134 (1999) 48–56. [20] D.C. Marcus, J. Liu, J.H. Lee, E.Q. Scherer, M.A. Scofield, P. Wangemann, Apical membrane P2Y4 purinergic receptor controls K+ secretion by strial marginal cell epithelium, Cell Commun. Signal. 3 (2005) 13. [21] M.P. Meyer, J.D. Clarke, K. Patel, A. Townsend-Nicholson, G. Burnstock, Selective expression of purinoceptor cP2Y1 suggests a role for nucleotide signalling in development of the chick embryo, Dev. Dyn. 214 (1999) 152–158. [22] T. Mori, T. Miyashita, K. Akiyama, R. Inamoto, N. Mori, The expression of P2Y1, 2, 4, and 6 receptors in rat endolymphatic sac epithelia, Neuroreport 20 (2009) 419–423. [23] V. Piazza, C.D. Ciubotaru, J.E. Gale, F. Mammano, Purinergic signalling and intercellular Ca2+ wave propagation in the organ of Corti, Cell Calcium 41 (2007) 77–86. [24] K.D. Peusner, D.K. Morest, The neuronal architecture and topography of the nucleus vestibularis tangentialis in the late chick embryo, Neuroscience 2 (1977) 189–207. [25] K.D. Peusner, C. Giaume, Ontogeny of electrophysiological properties and dendritic pattern in second-order chick vestibular neurons, J. Comp. Neurol. 384 (1997) 621–633. [26] C.L. Sage, D.C. Marcus, Immunolocalization of P2Y4 and P2Y2 purinergic receptors in strial marginal cells and vestibular dark cells, J. Memb. Biol. 185 (2002) 103–115. [27] E. Soto, R. Vega, A turbo pascal program for on line spike data acquisition and analysis, J. Neurosci. Methods 19 (1987) 61–68. [28] A. Szücs, H. Szappanos, A. Tóth, Z. Farkas, G. Panyi, L. Csernoch, I. Sziklai, Differential expression of purinergic receptor subtypes in the outer hair cells of the guinea pig, Hear. Res. 196 (2004) 2–7. [29] N.X. Tritsch, E. Yi, J.E. Gale, E. Glowatzki, D.E. Bergles, The origin of spontaneous activity in the developing auditory system, Nature 450 (2007) 50–55. [30] N.X. Tritsch, D.E. Bergles, Developmental regulation of spontaneous activity in the mammalian cochlea, J. Neurosci. 30 (2010) 1539–1550.