* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download A Yale geneticist and a Chinese lab are creating the Amazon.com of
Oncogenomics wikipedia , lookup
Ridge (biology) wikipedia , lookup
Biology and consumer behaviour wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Gene nomenclature wikipedia , lookup
Saethre–Chotzen syndrome wikipedia , lookup
Genetic engineering wikipedia , lookup
Epigenetics of human development wikipedia , lookup
Gene desert wikipedia , lookup
Gene expression programming wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
Gene therapy wikipedia , lookup
Minimal genome wikipedia , lookup
Gene expression profiling wikipedia , lookup
Genomic imprinting wikipedia , lookup
Genome (book) wikipedia , lookup
Genome evolution wikipedia , lookup
Microevolution wikipedia , lookup
Epigenetics in learning and memory wikipedia , lookup
Public health genomics wikipedia , lookup
Epigenetics of neurodegenerative diseases wikipedia , lookup
Nutriepigenomics wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Designer baby wikipedia , lookup
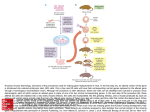
A Yale geneticist and a Chinese lab are creating the Amazon.com of medical research animals. Margot Sanger-Katz ’02 34 yale alumni magazine | may/june 2010 M ice like to bury marbles. If you give a laboratory mouse a handful of marbles, it will often bury a few into the bedding in its cage. But one particular mouse in Yale genetics professor Tian Xu’s lab will bury marbles all day long. That mouse is missing a gene. Having seen it at work, Xu and his colleagues now think the gene may be related to behavioral problems like obsessivecompulsive disorder. Another mouse of Xu’s appears to have the mouse equivalent of male-pattern baldness. It grows lovely white fur all over its body, but its head is bare. Studying the gene this mouse is missing could help scientists understand what causes some men to lose the hair on their heads. Tian Xu ’90PhD, who has developed an innovative system for easily producing mice missing single genes, is raising thousands of mutant mice in a laboratory complex in China—mice that don’t grow work. Texas A&M Health Science Center professor Richard Finnell, for instance, studies spina bifida, a birth defect that can lead to lifelong paralysis. In 2006, he developed a mouse with damage to a gene called FKBP8, which controls the development of the spinal column. (He calls the mouse “Stacey.”) He has used its descendants ever since in his research on the environmental factors that contribute to the birth defect. All told, scientists have so far succeeded in knocking out about a fifth of the 24,000 genes that have been mapped in the mouse genome. Xu’s break- fa c i n g pa g e : m a x o p p e n h e i m /g e t t y i m a g e s . t h i s pa g e : c o u r t e s y t i a n x u ’ 9 0 p h d . At Fudan University, Tian Xu created a simple system for disabling genes—a sort of assembly line for mutant mice. properly, mice that have kidney disease, mice with neurological problems, mice that lack sex appeal— in hopes that researchers around the world can use those mice to better understand human development and disease. His goal is a “functional map” of the mouse genome, linking every gene in the mouse to its function in the species. Such a tool has never been created for a mammal, and it could have major implications for human medicine. Humans and mice share about 99 percent of their genes, making mice, which are small, inexpensive to raise, and (relatively) short-lived, an ideal animal model for studying human disorders and testing possible treatments. Biologists have been studying mice for decades, using them to learn about physical and mental development, cancer, and heart disease, among other subjects. The scientists who developed the first system for disabling single genes in mice won the Nobel Prize in Medicine in 2007. Over the last two decades, scientists who needed mice missing specific genes have used gene splicing and other methods to engineer them for lab through, developed at Fudan University in Shanghai, where he has an adjunct faculty appointment, was in creating a simple and inexpensive system for disabling different genes quickly and efficiently—a sort of assembly line for mutants. In the first 18 months of his project, he’d already reached the 5,000-gene mark that took previous scientists 20 years. Instead of the painstaking work of splicing coded bits into mouse DNA, placing the manipulated DNA Tian Xu’s mutant mice glow pink under ultraviolet light, thanks to a custom-built piece of mobile DNA. m a r g o t s a n g e r - k at z ’02 is senior staff editor of the Yale Alumni Magazine. yale alumni magazine | may/june 2010 35 years, at a fraction of the cost required to complete the project the traditional way. “There’s a significant advantage to doing this stuff in China, because it’s just so cheap,” says Colin Fletcher, program manager for the National Institutes of Health’s (NIH) Mouse Knockout Project, Humans and mice share about which has funded part of Xu’s research. The project 99 percent of their genes. is also funding other scientists to knock out 8,500 genes in mouse stem cells using more-conventional with a disabled gene simply by mating two specially technologies; its goal is to make a complete “library” prepared mice. One of the mice has a piece of genetic of knockout mouse stem cells available to researchmaterial called a transposon, which can jump within ers. In China, Fletcher says, Xu’s work sees cost the genome of the reproductive cells, inserting itself savings in building construction and lab equipment, at random to disable an existing gene. The other but particularly in labor. A technician who would earn a salary of more than $30,000 in the United makes an enzyme that activates the jumping gene. Breed the two together, and each of their offspring States can be hired for about $4,500 in China. will be born with one inactive gene, a different gene he breeding facility occupies two of in each. The process doesn’t require molecular biolothe four buildings in a huge comgists, just skilled technicians who can care for the plex at Fudan University in Shangmice and run basic tests to determine which gene hai, the Fudan-Yale Biomedical is missing. Research Center. The first buildTransposons are common in plants and insects, ing, with about 25,000 square feet and scientists have used a similar technology to create mutants in insect species. But before Xu, no of mouse space, went up in just six months, Xu says, one had been able to find a transposon that would and the other buildings followed quickly. The fourth work efficiently in mammals. And, in a clever twist, and largest addition, still under construction, will into embryonic stem cells, and then implanting those cells into pregnant mouse wombs—a process that can take an individual lab as long as a year and cost more than $50,000—Xu can breed a mouse m o u s e i l l u s t r at i o n s : s h o n a g h r a e . T Tian Xu’s lab at Fudan University, in Shanghai, has created a system for producing thousands of mutant mice to help researchers investigate the functions of genes and develop treatments for disease. The artist’s conceptions on this and following pages illustrate some of the mutants the lab has already developed. 36 he has figured out a way to color-code the mice so that technicians can tell instantly whether a mouse is normal or a mutant: the mutant mice carry a gene that makes them glow pink under an ultraviolet lamp. The mutants are bred in a lab in China large enough to house 150,000 mice at a time. Overseen by about 150 Chinese lab technicians and scientists—and U.S. researchers via webcam—the lab is breeding its way through the mouse genome. Xu expects to disable nearly every mouse gene in a few yale alumni magazine | may/june 2010 add 100,000 square feet of space, most of it for mice. “This really made it possible to do it large-scale,” Xu says, marveling at the speed of the Chinese construction effort. The complex not only houses his project, but also aids Fudan faculty research and offers training for talented graduate students and postdocs—some of whom Yale may eventually attract to do research here. The collaboration with Fudan was Xu’s own suggestion, in part because he received his undergraduate training in genetics there. He grew up in China, Tian Xu’s big innovation was finding a special type of mobile DNA that could jump around the mouse genome and inactivate genes. To generate new mutants, his lab breeds two special mice. One is engineered to carry a copy of the mobile genecalled a transposon. The other produces an enzyme that activates the transposon. When you breed the two special mice together, each of their offspring will have one gene that’s been inactivated by the transposon, which inserts itself at random into the normal genetic material. Because of a special protein in the transposon, each of the resulting mice will appear pink under an ultraviolet lamp. TRANSPOSON ENZYME To raise more mice with the same mutated gene, the lab next breeds a pink mouse to a normal mouse. Because each mouse has two copies of every gene, and only one copy in the pink mouse is altered, only half of the resulting offspring will share the mutation. They can be easily spotted by their color. c h a r t : m a r k z u r o l o ’ 0 1 m fa . But mice with one mutated copy of a gene and one normal copy don’t always demonstrate mutations. To raise mice with both copies of the gene inactivated, the lab breeds pink mice from the same litter together. Some resulting offspring will be born missing both copies of the gene—and appear a darker pink. the son of intellectuals who suffered under the Cultural Revolution, and is quick to credit Yale for giving him opportunities—when he was a graduate student on fellowship who spoke little English and The lab is breeding its way through the mouse genome. when he was an assistant professor with a novel idea for a project on mice. But he says he is also eager to give back to Fudan, a school with highly regarded biology departments and numerous Yale connections. (Xu, who is the vice chair of the Yale genetics department and holds a prestigious five-year grant as a Howard Hughes Medical Institute investigator, is the director of the Fudan-Yale Center.) In its post-tercentennial push to globalize its research, Yale has created several such collaborations with Chinese universities. The projects fulfill President Richard Levin’s mission to “become a truly global university,” says Fawn Wang of Yale’s Office for International Affairs, who is tasked with setting up the Yale-China partnerships. But they also allow Yale researchers to complete major research on the cheap. Xu’s mouse buildings were built with grants from the Chinese government; Yale brought $1 million in NIH grant funding for the research, but didn’t raise donor gifts or contribute any money from its general fund. “This is such a world-class university,” Wang says. “They can use our name to apply for money.” Although costs are low for the lab at Fudan, Xu says he has been careful to ensure that the lab maintains the same high standards for animal care that would be employed in a Yale facility. He works at the facility for about one week out of every month and says the mouse areas are climate-controlled, roomy, and constantly monitored. Fudan is responsible for animal care, but Yale’s contract with Fudan specifies that it has to meet NIH animal care standards. As with other subcontractors, Yale does not yale alumni magazine | may/june 2010 37 undertake on-site inspections. But a representative of Yale’s Institutional Care and Animal Use Committee toured the facility with President Levin when it opened, Xu says, and pronounced it better than some animal labs in New Haven. He considers it an important visit, “so we were not attacked for running a sweatshop.” the diagnostic work begins even before the mutant mice are born. About 15 percent of genes appear to be so vital to survival that mice without them die in utero. Some of those that survive will have visible problems: they won’t grow normally, or they have patchy fur, or they grow tusks. Others require more advanced testing. Every mouse has its blood reeding the mice is just the first step. Because such a small portion of the genome is currently understood, Xu’s next project is figuring out what effect each disabled gene will have on the development, health, and behavior of the mice. And diagnosing the problems is a complex business, because Xu’s method of knocking out genes is random. Unlike the molecular biologists who disable a selected gene because they think it may be related to diabetes or cancer, Xu has The mice get colonoscopies and CT scans. Motor control is tested on tiny balance beams. no way to control which genes will be deactivated by his breeding process. So when each mouse is born, he needs to test it for a whole battery of different abnormalities. Back at Yale, Xu is at work creating the “most advanced mouse hospital on earth.” Parts of that hospital are already functional, in his current lab at the Boyer Center for Molecular Medicine. A miniature CT (computed tomography) scanner sits in the corner of a laboratory room, along with a hot plate (used to detect pain response) and other mousetesting equipment. But the mouse hospital will soon be a part of Yale’s new West Campus, where substantial mouse vivarium space will be devoted to Xu’s project. “I was trained as a PhD in genetics to become a mouse doctor,” Xu jokes. Yun You, an associate research scientist at Yale who is managing the mouse testing effort, says disorders, and trouble with breeding. Xu hopes this method will reveal many more genes of medical importance than would have been discovered the old way, by guesswork. As the lab learns more about the function of each gene, Xu and his colleagues will publish scientific papers and enter the information about each mouse into a central Internet database. That website is already live with about 1,200 known genes. Scientists can use the site as a sort of catalog, then contact Xu with an order if a particular mutant would aid their research. Xu wants to create a shopping-cart system, so that scientists can search for traits and genes and order frozen embryos or live mice in a few simple steps. “They’re all available,” says Jon Soderstrom, managing director of the Yale Office of Cooperative Research, who is helping Xu patent the mice and set B Some mutant mice develop obvious problems, like baldness or difficulty absorbing nutrition; others require testing to identify the functions affected when a given gene is disabled. This research is done at Yale. At first, scientists thought one particular mutant (at center) was sterile. After further investigation, they found that the mouse’s reproductive biology was fine—but females refused to mate with it. 38 yale alumni magazine | may/june 2010 drawn and its heart rhythm scanned. The mice get colonoscopies, X-rays, and CT scans. Motor control is tested on tiny balance beams. Mouse memory is tested using special mazes. Behavior tests look for depression, fearfulness, obsessive-compulsive up a for-profit corporation to manage the mouseselling effort. “They’ll all be cataloged, and you’ll be able to go online and click through and put your order through.” That concept is similar to a website the NIH has built for its knockout mouse project, but is vastly different from how knockout mice were obtained for research in the past. Until recently, there has been no comprehensive database listing knockouts and because his method creates live breeding pairs. The NIH library will provide modified stem cells that are many complex steps away from living research animals, because scientists must transfer them into mouse embryos that they then breed for several generations. Research has also shown that different mutant technologies can yield subtly different effects, and Xu’s mice may be favored by certain scientists. “Our no reliable way for scientists to put in requests, even for those mice that were ready. Though Yale’s plan is to make money from mouse distribution, the project’s overall goal is to make mice widely available, at lower prices than those of most labs that currently offer mutant mice to researchers. Stefan Somlo, a nephrologist who works across the street from Xu, read an early published report about Xu’s mutants, and found a mouse that was perfect for his own work on a common inherited kidney disease that causes cysts and kidney failure. He hopes the transposon-created mouse will help him find out whether therapies that turn the gene on and off will influence the progress of the disease. “This is an enabling technology for us,” he says. work is complementary,” says Kent Lloyd, a veterinarian and research physiologist at the University of California–Davis who is working on the NIH knockout project. Xu wants to create a shoppingcart system, so that scientists can search for traits online and order live mice in a few simple steps. Xu’s mice may help scientists understand the biological underpinnings of behavior. His lab at Yale is looking for mutants that demonstrate symptoms of mental illness, like fearfulness, depression, or obsessive-compulsive behaviors. Researchers have already found one mouse that obsessively buries marbles in its cage. Xu is also carving out his own project in the mouse genome. He knows that most of the mice he creates will benefit other researchers, but he’s taken an interest in a few mutants they’ve already uncovs NIH scientists rush towards a ered. One particularly promising mutant has trouble similar goal—a complete library absorbing nutrients in its digestive system. It is born of knockout mice—it is unclear with chronic diarrhea and ultimately dies after sufwhether Xu’s mice will become fering many of the same symptoms seen in starving the favored research animals or children. Xu thinks the mouse could serve a double simply one of several options for value. By determining how to improve the effiscientists. But if it works, Xu’s massive experiment ciency of its digestive system, Xu hopes he can help could serve as a demonstration project for how develop treatments to mitigate starvation in parts to catalog an entire genome quickly. He’s already of the world where food is scarce. He also hopes the shown that a similar transposon technology can mouse’s inefficient digestive system will help him be used to create mutant rats, and he believes it find ways for humans who eat junk food and live could work in other mammal species that may be sedentary lives to avoid the ravages of diabetes and preferable for some kinds of research. Moreover, Xu heart disease. It’s a very American question for a says his mice are likely to be less expensive to use Chinese mouse. A yale alumni magazine | may/june 2010 39