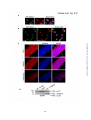
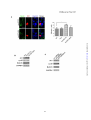
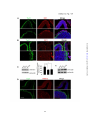
* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Transcription Factor Positive Regulatory Domain 4 (PRDM4) recruits Protein Arginine
Tissue engineering wikipedia , lookup
Cell growth wikipedia , lookup
Signal transduction wikipedia , lookup
Cytokinesis wikipedia , lookup
Extracellular matrix wikipedia , lookup
Cell encapsulation wikipedia , lookup
Cell culture wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Histone acetylation and deacetylation wikipedia , lookup
List of types of proteins wikipedia , lookup