* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download The action potential and the synapses
Theories of general anaesthetic action wikipedia , lookup
Model lipid bilayer wikipedia , lookup
Cell-penetrating peptide wikipedia , lookup
List of types of proteins wikipedia , lookup
Mechanosensitive channels wikipedia , lookup
Western blot wikipedia , lookup
Cell membrane wikipedia , lookup
SNARE (protein) wikipedia , lookup
Node of Ranvier wikipedia , lookup
Endomembrane system wikipedia , lookup
Membrane potential wikipedia , lookup
Action potential wikipedia , lookup
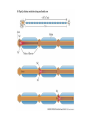
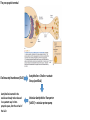
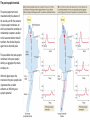
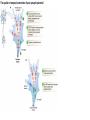
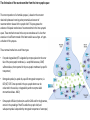
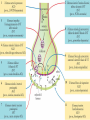
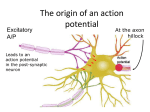
The action potential and the synapses Lesson nr. 16 - Psychobiology Phases of the action potential The action potential consists in a sudden and transient modification of the neuronal membrane potential that can be divided into 3 distinct phases: rising phase (strong depolarization) descent phase (strong hyperpolarization) recovery phase (mild depolarization) The action potential is triggered because in the membrane, is reached the threshold value (-55 mV) that allows the opening of voltage-gated Na + channels + 56 mV - 89 mV Duration and amplitude of the action potential In addition to the 3 phases described above, The potential is also characterized by two important parameters: Duration. In most cases, it corresponds to about 4-6 msec and depends by the kinetics of opening and subsequent obliteration of the voltage dependent Na + and K + channels, and thus by the specific channel subtypes (isoforms) expressed on neuronal membranes. Amplitude. It corresponds to the potential variation between the maximum and the minimum peak. It does not depend directly from the channels, but by the concentration of free ions present in the intraand extra-cellular space. The consequence of this is that all the potential generated in the same neurons have always an amplitude similar, and that for the neuron, the only possible alternative is the emission or non-emission of an action potential (all-or-none phenomenon). Self-regeneration of the action potential The action potential takes place in a specific region of the membrane region, very small in size, and therefore represents a local phenomenon. However, the action potential also strongly influences the distribution of electrical charges in its immediate vicinity generating a depolarization gradient that will bring the voltage-gated channels in the immediate surroundings to reach their threshold, allowing the propagation of potential. Modulation of the nerve information As said, the action potential is defined as an all-or-none phenomenon, therefore, is not possible to modulate its intensity as is the case for example with the volume of our voice. It arises at this point the problem of how neurons can modulate the intensity of nervous stimuli. The solution adopted is the variation of the frequency of emission (of potential trains) Modulation of the nerve information The synapse The chemical synaptic junctions have the task of transforming the electric information into chemical information and are composed of: Pre-synaptic junction Synaptic cleft Post-synaptic junction Neurotransmitters They are molecules that mediate the unidirectional transmission of the nerve stimulus in the chemical synapses. Are conventionally divided into: The low molecular weight neurotransmitters are synthesized in the cytosol of the presynaptic termination and, subsequently, by means of active transport, are absorbed inside of the numerous vesicles in the synaptic terminal. When a signal reaches the synaptic terminal, a few vesicles at a time, release their neurotransmitter into the synaptic cleft. This process generally takes place over a period of one millisecond. classical neurotransmitters (small, electric or rapid metabolic reactions) Neuropeptides (large, more complex functions, slow dynamic and continuous) The neuropeptides, instead, are synthesized as part of large protein molecules by ribosomes of the neuronal soma. These proteins are immediately transported into the endoplasmic reticulum and then inside of the Golgi apparatus, where two changes occur. At first, the protein from which the neuropeptide is cleaved enzymatically will be divided into smaller fragments, some of which constitute the neuropeptide as such or a precursor thereof; later, the Golgi apparatus Bundling neuropeptide in small vesicles that bud from it. Thanks to the axonal flow vesicles are transported to the ends of the nerve endings, ready to be released into the nerve terminal with the arrival of an action potential. Typically the neuropeptides are released in much smaller quantities than the classic neurotransmitters, but this is offset by the fact that neuropeptides are much more powerful. The pre-synaptic terminal Choline-acetyl-transferase (ChAT) Acetylcholine inserted in the vesicles and ready to be released in a quantum way in intersynaptic space, after the arrival of the Ca2 + Acetylcholine = Choline + acetate Group (acetilCoA) Vesicular Acetylcholine Transporter (VAChT) + vesicular proton pump The post-synaptic terminal The post-synaptic terminal is characterized by the absence of the vesicles, and for the presence of post-synaptic membrane, on which are placed the ionotropic or metabotropic receptors sensitive to the neurotransmitter that will transform the chemical impulse again into an electrical pulse. The space below the post-synaptic membrane is the post-synaptic density, an aggregate of proteins, enzymes, etc ... When the ligand opens the channels on the post-synaptic side it generates the so-called excitatory or inhibitory postsynaptic potential The spatial or temporal summation of post-synaptic potential The elimination of the neurotransmitter from the inter-synaptic space The correct operation of a chemical synapses, is based on the constant relationship between incoming action potentials and amount of neurotransmitter released in the synaptic cleft. This presupposes the existence of disposal mechanisms of neurotransmitter in the inter-synaptic space. These mechanisms need to be very accurate because it is clear that excessive or insufficient removal of the latter would cause a hypo- or hyperactivation of the synapses. These removal mechanisms are of three types: • Enzymatic degradation (NT is degraded by enzymes placed on the outer face of the postsynaptic membrane, i.e. acetylcholinesterase, COMT, sulfotransferase; then reported in the pre-synaptic membrane by specific transporters) • Retrograde uptake (re-uptake by a specific retrograde transporter, i.e. DAT, NET, SERT. Once reported in the pre-synaptic button can be reinserted in the vesicles, or degraded by another enzyme called monoamine oxidase - MAO) • Extrasynaptic diffusion (mechanism used for GABA and for the glutamate, consist in the spreading of the NT outside the synaptic cleft, and subsequent uptake is dependent by retrograde transporters of astrocytes) Electric synapse Also called gap junctions, allowing fast and bidirectional transfer of nerve information, connecting two cells as if they were a single cell, called metabolic syncytium.