* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download The pattern of ocular dominance columns in macaque visual cortex
Emotional lateralization wikipedia , lookup
Time perception wikipedia , lookup
Neuroplasticity wikipedia , lookup
Aging brain wikipedia , lookup
Human brain wikipedia , lookup
Neuroeconomics wikipedia , lookup
Neuroesthetics wikipedia , lookup
Microneurography wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
C1 and P1 (neuroscience) wikipedia , lookup
Cortical cooling wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
Inferior temporal gyrus wikipedia , lookup
Hierarchical temporal memory wikipedia , lookup
The Pattern of Ocular Dominance Columns in Macaque
Visual Cortex Revealed by a Reduced Silver Stain
SIMON LEVAY, DAVID H. HUBEL AND TORSTEN N . WIESEL
Department of Nezc?obiology, Hn,-vu?d Medicrrl School, 25 Shattitck Strecrt,
Boston, Mnsscichicsetts 021 1 5
ABSTRACT
A pattern of alternating dark and pale bands was observed in the
striate cortex of the macaque monkey. The bands, which ran parallel to the surface, were seen in tangential sections stained with a reduced silver method for
normal fibers and were most clear in layer 4C a, immediately deep to the line of
Gennari. The dark bands were about 300 F wide and showed blind endings and
bifurcations. The light bands were about 50 p wide and did not branch or terminate within area 17.
Because the dark bands were similar in width to the bands of terminal degeneration which have been shown to result from single-layer lesions of the lateral
geniculate body, i t seemed possible that they corresponded to ocular dominance
columns. To test this idea, the boundaries of ocular dominance columns were
marked in a physiological experiment: tangential electrode penetrations were
made in an anesthetized monkey and, a s the electrode was advanced horizontally
in the fourth layer, the eye preference of single units and of the background activity was monitored. Small electrolytic lesions were placed at the points where a
change in eye preference occurred. The brain was subsequently fixed, sectioned
tangentially and stained with the silver method. All the lesions- a total of 12
-fell directly on the pale bands. Moreover, the electrode had not passed over
any pale bands without a lesion being placed. It was concluded that the dark
bands do correspond to single ocular dominance columns and the pale bands to
the boundaries between columns.
The banding appearance i s due to a greater density of tangential fibers within
columns than at the borders of columns. These tangential fibers are in part the
preterminal arborizations of geniculocortical axons, since some of them have been
shown to degenerate after geniculate lesions.
The ocular dominance columns were mapped for most of the striate cortex,
using serial tangential sections stained with the silver method. The overall pattern was similar in several monkeys, though the details of the branching arrangements varied from animal to animal. The columns met the 17-18 border
at right angles. On the outer surface of the hemisphere the columns converged
from the 17-18 border, turned medially with repeated fusions of columns, and
streamed over the lip of the calcarine fissure. In the roof of the fissure they met a
second system of columns oriented parasagittally. In terms of the visual field, the
columns ran roughly horizontally for the central 10" of the field, and circumferentially beyond that. The columns were not mapped in the stem of the fissure,
the area corresponding to the far periphery of the field.
The constancy of column width across the cortex probably allows a functional
matching between ocular-dominance and orientation columns.
The monkey's striate cortex is subdivided
into two independent sets of columns, one
for eye preference and one for preferred
stimulus orientation, and these columnar
systems are superimposed on a n orderly
projection of the visual field (Hubel and
Wiesel, '68, '74a). The ocular dominance
J. COMP. NEUR.,159: 559-576.
columns are generated by a spatial segregation of the afferents from the ipsilaterally and contralaterally innervated layers of
the lateral geniculate nucleus. By placing
lesions in single layers of the lateral geniculate nucleus Hubel and Wiesel ('72) were
able to show that the afferent terminals in
559
560
SIMON L E V A Y DAVID H . H U B E L A N D TORSTEN N. WIESEL
layer 4 were arranged in parallel and alternating left and right eye bands. This method of demonstrating the columns was limited by the necessity of making the geniculate
lesions small enough to be entirely restricted to a single layer, so that the area of cortex over which the columns could be reconstructed was also rather small. The study
therefore left two intriguing questions unanswered: did the columns form a pattern
that was constant from animal to animal,
and, if so, what relationship did this pattern have to the visual field representation?
During the course of a n investigation intended to reveal the anatomical basis of
orientation columns, we recently noticed a
pattern of parallel bands in the fourth layer of the cortex. The bands were seen in
tangential sections stained with a reduced
silver method for normal fibers. In this
paper we describe this banding pattern and
show that it corresponds to the ocular dominance system, as defined physiologically.
By making reconstructions from serial sections, we show that the ocular dominance
columnar system does form a constant overall pattern, with a definite relationship to
the visual field map.
METHODS
Six macaque monkeys (five Rhesus monkeys, Macaca mulatta, and one crab-eating macaque, M. fascicularis) were used in
the present study. Two of the animals were
normal young adults; the other four had
a surgically-induced strabismus. All four
strabismic monkeys were shown by behavioral testing to have normal visual acuity in
both eyes, but lacked binocular fusion (work
to be published separately).
At the termination of physiological experiments, most of which were not relevant
to the present study, the animals were perfused with 10% formol saline, and blocks
of striate cortex, or the whole occipital lobe,
were cut in serial frozen sections oriented
tangentially to the pial surface. To demonstrate the banding pattern in the fourth
layer, sections were stained with a slight
modification of Liesegang's reduced silver
method (Jones, 'SO), itself a variation of
one of Cajal's silver methods. The sections
were washed, left overnight in 50% alcohol
containing sixteen drops of concentrated
ammonium hydroxide per 100 ml, washed
again, and silvered in a 0.75% silver ni-
trate solution at room temperature for six
hours. Without washing, the sections were
reduced in the following mixture:
50r; w/v acacia powder in water:
0.5C; hydroquinone:
l o 1 , silver nitrate:
80 ml
40 nil
3 ml
The reduction was allowed to proceed, in
subdued light, until the white matter was
completely black (20-30 min). The sections
were then rinsed and mounted on albumenized slides. This method gives an intense
staining of large and medium sized axons,
and leaves all but the largest cell bodies
unstained.
In the experiments designed to test the
relationship between the banding pattern
and the ocular-dominance system, tangential electrode penetrations were made in
the striate cortex of anesthetized, paralyzed
monkeys, using recording techniques previously described (Hubel and Wiesel, '74a).
We used electrodes with uninsulated tips
of 25-35 p , which allowed a continuous
monitoring of unit activity as well as the
unresolved background activity characteristic of the fourth layer. When the electrode
tip was within the fourth layer, the electrode was advanced in 20-50 p steps, and
the eye preference of the units and of the
background activity was noted. At the points
where eye preference changes, i.e., at the
borders of ocular dominance columns,
small electrolytic lesions were placed (1 pA
for 1 sec, electrode negative, producing lesions 30-50 p in diameter). A t the conclusion of the experiment the animal was perfused and a block of cortex containing the
electrode tracks was sectioned tangentially
and stained with the reduced silver method.
RESULTS
Architecture of the fourth layer in
uertical sections
The pattern of bands to be described in
this paper is visible only in tangential sections of the cortex. To clarify the structures
involved in forming this pattern, however,
we shall first describe the appearance of
the fourth layer in vertical sections stained
with the Nissl and reduced silver methods
(fig. 1). The nomenclature of the layers follows that used by Lund ('73) and differs
slightly from that used by Hubel and Wiesel ('72) in that layer 4C is enlarged at the
expense of 4B. Layer 4C is subdivided into
two parts. The upper part, layer 4C C I , lies
TOPOGRAPHY OF OCULAR D O M I N A N C E C O L U M N S
56 1
3
5
562
SIMON LEVAY, DAVID H . HUBEL AND TORSTEN N. WIESEL
immediately under the stria of Gennari. It
is moderately rich both in cells and in tangential fibers and receives the afferents
from the magnocellular layers of the geniculate (Hubel and Wiesel, '72). Layer 4C p
is more densely cellular and contains fewer
tangential fibers; it receives the bulk of the
afferents from the parvocellular layers of
the geniculate. Layer 4B contains a dense
tangential fiber plexus, the stria of Gennari, and a few large cells; it receives no
direct input from the geniculate. Layer 4A
lies immediately above the stria and receives a second, less dense tier of terminals
from the parvocellular layers of the geniculate.
Besides the tangential fibers, there are
also fascicles of vertically-oriented fibers
passing through the fourth layer. These
fiber bundles (fig. l b ) consist mainly of the
descending axons of pyramidal cells in the
supragranular layers, with a smaller contribution from the ascending collaterals of
more deeply-lying cells and geniculate
axons ascending to layer 4A.
Tangential sections - the banding
in layer 4C
Because the surface of the cortex is convex, sections cut tangential to the pial surface show the cortical layers as concentric
rings, with the deepest layers in the center
and the pial surface at the outside. When
such sections were stained with the reduced
silver method a pattern of bands could be
seen in the fourth layer (fig. 2). This pattern was seen most readily when the slides
were viewed at low magnification - for example, by projecting them in a photographic enlarger. The pattern consisted of a system of dark-staining bands, each about
300 p wide, alternating with pale bands
that were much thinner, about 50 p wide.
The borders between light and dark bands
were not at all sharp, so that the apparent
width of the light bands varied somewhat
with different staining intensities and with
different conditions of viewing the sections.
The bands were most clearly visible in layer 4C a, just below the line of Gennari, but
could usually also be made out in 4C p, and
sometimes also in 4A and 5.
Identification o f t he dark bands w i t h
single ocular dominance columns
The fact that the dark bands were the
same width as the bands of degeneration
resulting from laminar lesions of the geniculate (Hubel and Wiesel, '72) suggested to
us that we might be looking at the pattern
of ocular dominance columns. This possibility was reinforced by observation of the pattern of branching of the bands. The thin
pale bands were never seen to branch, nor
did they terminate except at the boundary
of area 17. The wide dark bands, on the
other hand, often branched or ended blindly. Fusion frequently occurred between
every second dark band, but not between
immediately adjacent dark bands. This
manner of branching stood out clearly when
the entire banding pattern was reconstructed (see below), and it indicated to us that
there were two sets of dark bands forming
two separate, non-communicating systems.
In terms of ocular dominance columns,
these two systems would correspond to columns for the left and right eye respectively,
while the thinner pale bands would represent the boundaries between adjacent
columns.
We therefore set out to test this idea directly by marking the column boundaries
with lesions in a physiological experiment
and subsequently comparing the positions
of the lesions with the banding pattern in
the fixed and stained brain. More or less
tangential penetrations were made through
the cortex in order to cross as many columns as possible while the electrode remained within the fourth layer. The layer
was recognized during the experiment by
the prominence of units lacking stimulus
orientation specificity, the small size of the
receptive fields, the lack of scatter in receptive field position (Hubel et al., '74), the
strict monocularity of single units, and
the characteristic rich monocularly-driven
background activity of many small unresolved units.
Four penetrations were made, all in the
right hemisphere. The first three were made
in a mediolateral direction, a short distance behind the 17-18 border. In each of
the first two penetrations the electrode was
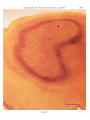
Fig. 2 Low-power micrograph of a tangential
section of striate cortex stained by the reduced silver method. The dark-staining ring is the line of
Gennari (layer 4B). In layer 4C, the area within the
ring, there are alternating dark (fiber-dense) and
light (fiber-poor) bands. In the area marked with
a n asterisk, the section grazes layer 5; here the
bands are not visible. Bar = 1 mm.
TOPOGRAPHY OF OCULAR D O M I N A N C E C O L U M N S
Figure 2
563
564
SIMON LEVAY, D A V I D H . H U B E L A N D T O R S T E N N . WIESEL
angled rather too steeply, so that only one
change in eye preference was encountered
while the electrode remained within the
fourth layer. Both these transition points
were marked with lesions. In the third penetration the angle was better, and within
layer 4 five successive changes in eye preference were marked. For the fourth penetration the electrode was turned through
90" and directed posteriorly, near the medial edge of the hemisphere. In this penetration three changes in eye preference
occurred at normal intervals, but then there
was an unusually long sequence, almost
2 mm in length, during which all cells
were driven strongly by the left eye, but
with faint background activity from the
right eye. A reversal from left to right eye
followed, and finally there was a fifth reversal, from right to left, before the electrode
left the fourth layer.
When the part of the cortex containing
the electrode tracks was subsequently examined in serial tangential sections, all
twelve lesions were found to lie directly on
the thin pale bands. A section from this
series is illustrated in figure 3. It contains
two of the lesions, those made during the
first two penetrations. The lesions were easily identified at high magnification. With
dark-field illumination the electrode tracks
were also visible.
Because the sections were cut tangentially, the reconstruction of the banding
pattern for the whole block was a relatively
easy task. The sections were projected one
at a time in a photographic enlarger, and
wherever layer 4 appeared in the section
the thin pale bands and the lesions were
traced onto a master diagram (fig. 4a).
The reconstruction shows, first, that all the
lesions were placed on the narrow pale
bands (represented a s solid lines in this and
subsequent reconstructions). It also shows
that in each of the two penetrations in
which several consecutive lesions were
made no pale bands were passed over without a lesion being placed. The experiment
therefore demonstrated that the broad dark
bands do correspond to single ocular-dominance columns and that the narrow pale
bands are the boundaries between columns.
From the physiology, each of the columns,
between lesions could be assigned either to
the right or to the left eye. The consistency
of the assignments across the reconstruc-
tion is shown in figure 4b, in which the
columns belonging to the left (contralateral) eye have been shaded. It can also be
seen that the two sets of columns, corresponding to the right and left eyes, are
topologically similar structures: both are
elaborately branched, and both have some
isolated, blind-ending segments. The areas
of the two columnar systems in the reconstruction were measured, and found to be
almost equal: the contralateral columns occupied 2 % more area than the ipsilateral.
The relationship between the bands and
ocular dominance columns has been confirmed in several other animals which were
examined either to clarify the organization
of the visual field representation in the
fourth layer (Hubel et al., '74) or to investigate the effects of visual deprivation on
the columns.
Structural basis of t h e banding pattern
Since the silver method used here stained
only nerve fibers, the appearance of pale
and dark bands presumably reflected a difference in fiber density. Figure 5 shows, at
higher magnification than in the previous
illustrations, a single pale band flanked by
parts of the two adjacent dark bands. At
this magnification the pale band is not at
all striking as a discrete entity. One can
see, however, that the tangentially oriented
fibers are sparser within the pale band than
in the dark bands. There is also a suggestion that the vertical bundles of fibers, seen
a s dense round clumps in the figure, are
somewhat thinner within the pale band.
We re-examined the Fink-Heimer preparations of the cortex used in the laminarlesion study (Hubel and Wiesel, '72) to see
whether geniculate axons could be contributing to the banding pattern. With lesions
of either the magnocellular or the parvocellular layers of the geniculate, one finds
many degenerating preterminal axons oriented tangentially in layer 4C (fig. 6). Although some of these degenerating fibers
crossed columnar borders (usually running
clear across the adjacent column as if to
arborize in the next column belonging to
the same eye), a much greater number were
confined to single columns. We therefore
believe that the geniculate axons themselves may make a substantial contribution
to the banding pattern visible in reduced
silver preparations. But there may also be
TOPOGRAPHY OF OCULAR DOMINANCE COLUMNS
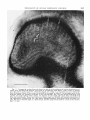
Fig. 3 Tangential section from the brain in which the boundaries of ocular dominance colu m n s were marked with lesions. Note that in comparison with figure 3 this section i s deeper
into the cortex, so t h a t layer 4C, where t h e b a n d s are visible, is a n a n n u l a r area j u s t within the
line of Gennari. Most of the center of the field is occupied by layer 5. I n t h e upper part of the
field the b a n d s a r e running vertically in the micrograph. The two pale b a n d s indicated with
arrows are each marked with a lesion: the lesions are t h e small pale spots about one-half inch
from the arrowheads. These two lesions are those made on the first and second penetration of
the experiment (compare fig. 4a). T h e m a n y sharply-bordered, dead-white holes are blood vessels; the line a t bottom right is a knife mark, and the large white mark is a tear i n the section.
Bar = 1 mm.
565
566
0
E
a
i
L
I
I
I
I
I
Fig. 4a Reconstruction of the banding pattern (solid lines = pale bands), electrode tracks (arrows)
and lesions marking the borders of ocular dominance columns (black dots). All twelve lesions, made in
four penetrations, coincide with the pale bands. Note also that for much of the fourth penetration the
electrode was running along a dark band, close to its edge (see text).
Fig. 4b The same reconstruction, indicating the eye preference of columns crossed by the electrode. The left-eye (contralateral) ocular dominance columns have been shaded. Dashed line: 17-18
border. Bar = 5 mm. Posterior is down in this figure.
TOPOGRAPHY OF OCULAR DOMINANCE COLUMNS
a segregation according to eye of other fibers, particularly of the axon collaterals of
the spiny stellate cells intrinsic to the fourth
layer.
In the visual cortex of kittens raised with
a n artificial squint there is physiologically
a more pronounced segregation of cells by
ocular dominance than is found in the normal cortex (Hubel and Wiesel, ’65). Monkeys raised with squint were therefore examined in the present study in the hope
that the columns might be revealed not only
in the fourth layer but throughout the entire thickness of the cortex. In fact, no clear
difference was found between the appearance of the columns in normal and in
squinting monkeys.
Mapping the ocular dominance system
Reconstructions of the columnar system
for most of the striate cortex were prepared
from serial tangential sections stained with
the reduced silver method. To make these
reconstructions understandable it is necessary to describe the shape of the striate cortex and the manner in which the visual
field is mapped onto it. We base the following summary of the topography on the findings of Talbot and Marshall (’41) and Daniel and Whitteridge (‘61).
Much of the striate cortex lies on the
smooth outer surface (operculum) of the
occipital lobe (fig. 7a). This area contains
the representation of the most central part
of the contralateral visual field. The foveal
representation lies laterally (fig. 7b), and
the horizontal meridian runs lateromedially, reaching 8 ” of eccentricity at the midline, where it doubles back along the roof
of the calcarine fissure. The vertical meridian is represented at the 17-18 border
(interrupted lines in figs. 7a,b): below the
fixation point it runs upward and medially
a short and variable distance behind the
lunate sulcus; above the fixation point it
runs downwards and medially round the
inferior rim of the operculum. The representation of the central part of the vertical
meridian by a C-shaped curve on the cortical surface is in part a reflection of the
greater magnification factor (the distance
on the cortex representing one degree of
visual field) for the central part of the field.
This expansion in magnification is illustrated in figure 7b, in which the representations of the horizontal meridian and of
567
parallel horizontal lines one, three, and
six degrees above and below the meridian
are sketched onto the opercular surface.
These can be seen to diverge as the vertical
meridian is approached.
At the midline, area 17 is folded back
under itself to form the roof of the calcarine
fissure, a triangular piece of cortex underlying the medial part of the operculum (fig.
7a, dotted line). Parasagittal sections (figs.
7c-e) show that at the superior and inferior
margins of this triangular region the cortex
curls under to form a third and even deeper
level, occupying the same triangular area
a s the second but consisting of two separate
leaves. Where the two leaves meet, they
turn anteriorly and form the banks of the
“stem” of the calcarine fissure. Medially
the roof of the fissure is broad and the stem
short, but laterally the roof becomes progressively narrower and the stem longer
(figs. 7c-e). The horizontal meridian runs
laterally, roughly across the middle of the
roof, and at its lateral end (representing
about 14” of eccentricity according to Talbot and Marshall, ’41) it turns anteriorly
and runs along the blind lateral end of the
stem. Thus the temporal crescent lies entirely within the stem of the fissure.
In order to reconstruct the columnar
pattern over as large a n area as possible,
the occipital lobe was sectioned in a plane
tangential to the operculum. This plane
(the plane of figure 7a) was tangential to
three levels of cortex - the exposed surface, the roof of the calcarine fissure, and
the two leaves joining the roof to the stem.
The only parts of the cortex not mapped
were the stem of the fissure, representing
the most peripheral part of the field, and
the regions of high curvature joining the
operculum with the roof of the fissure
medially, and joining the roof to the two
leaves.
Reconstructions of the ocular dominance
columns for these three levels of cortex are
shown in figure 8. The reconstructions are
from a single animal. In another animal
less perfect reconstructions were made for
the same areas on both sides of the brain,
and in four further animals partial reconstructions were made, confined to the
operculum. Casual observations of the directions of the columns in various parts of
the cortex have been made in a number of
other animals. Though each pattern was
568
SIMON LEVAY, DAVID H. HUBEL AND TORSTEN N. WIESEL
Fig. 5 High-power micrograph of a single pale band (running horizontally across the field
between the arrows) and parts of the two flanking dark b a n d s (top and bottom of field), i n tangential section. The density of tangential fibers within the pale band is lower than i n the dark
bands. The bundles of vertically-oriented fibers, seen a s dense clumps i n cross-section, a r e possibly also a little thinner (fewer fibers per bundle) in the pale bands. Bar = 100 u.
TOPOGRAPHY OF OCULAR DOMINANCE COLUMNS
569
5 70
SIMON L E V A Y . DAVID H . H U B E L AND TORSTEN N . WIESEL
b
'
I
n
/
L.S.
e
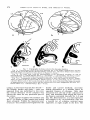
Fig. 7 Topography of striate cortex i n the macaque monkey.
Fig. 7a Posterolateral view of the brain showing the 17-18 border (dashed line) and the
extent of the roof of the buried calcarine fissure (dotted line). The three oblique lines indicate
the levels of the parasagittal sections shown in figures 7c, d and e.
Fig. 7 b The same view, with the representation of the horizontal meridian ( 0 ) and of
horizontal lines 1 , 3 and 6 degrees above (
and below - ) the meridian. T h e fovea is represented laterally, where the horizontal meridian meets the 17-18 border (vertical meridian).
Fig. 7c-e Parasagittal sections (medial to lateral) to show the extent of area 1 7 (black).
There are three levels of cortex lying in parallel planes: (1) the operculum, (2) the roof of the
calcarine fissure and ( 3 ) the leaves joining the roof to the stem. The stem of the fissure is oriented perpendicular to the other levels, and the columns have not been mapped for this area. L.S.:
lunate sulcus. C.F.: calcarine fissure. Bar = 5 mm.
+
unique, in the sense that the fine details the islands, breaks and forks - were not
repeated from one individual to the next,
the overall direction of the columns was
always the same for any particular part of
the cortex.
In the regions mapped, the columns met
the 17-18 border at right angles and ended
there abruptly. Within the opercular part
of area 17 the columns converged from the
border and turned medially, accommodating themselves to a smaller space by
repeated fusions of columns. They then
streamed over the lip of the calcarine fissure, and doubling back along the roof of
the fissure continued laterally for 1-2 mm
(fig. 8b). Here however, at a line representing roughly 9-10' of eccentricity, they met
a second set of columns oriented parasagittally, at right angles to the first. The
TOPOGRAPHY OF OCULAR DOMINANCE COLUMNS
boundary between the two systems was not
a complete discontinuity but a series of
small-scale fusions, loops and turns.
The parasagittally oriented columns in
the more lateral part of the roof of the fissure were continued onto the floor of the
superior and inferior limbs, which formed
the third plane of reconstruction (fig. 8c).
They then disappeared over the lips of the
stem of the fissure, heading for the 17-18
border, which runs mediolaterally near the
base of the stem.
The first set of columns, situated on the
operculum and extending a short distance
onto the roof of the calcarine fissure, ran
roughly along the cortical representation
of horizontal lines in the visual field. This
may be seen by comparing figure 8 a with
figure 7b. Within the foveal area there were
a few loops which were not continuous with
the main system. The second set of columns,
within the calcarine fissure, were oriented
roughly along circumferences in the visual
field.
The width of the columns was remarkably constant. In particular there was no
obvious systematic variation in different
parts of the cortex: for example, it was
similar for the foveal area as compared with
the most peripheral area examined. There
was some variation from brain to brain, the
range being 250-350 p. This amount of
variation might well be caused by varying
degrees of shrinkage during processing of
the tissue. In the brains containing electrode tracks the shrinkage could be estimated by comparing the distances between
lesions with the same distances as read
from the electrode advancer; this gave a
value for the shrinkage of about 2 5 % , so
that the width of the columns in life would
be about 3 0 0 4 5 0 p .
DISCUSSION
It comes as a surprise that the ocular
dominance system, which was originally a
physiological concept, and which was later
demonstrated anatomically by an elaborate
technique of reconstructing terminal degeneration after laminar lesions of the geniculate, may be visualized and mapped
by a method as simple and old-fashioned as
a Cajal silver stain. The columns are visible
in reduced silver preparations because a
narrow band at the borders between adjacent columns contains a slightly lower
571
density of tangential fibers than is found
within the columns proper. We attach no
functional significance to the fact that the
column boundaries, as seen in these preparations, have an appreciable width. We
suggest that the geniculocortical axons
themselves contribute to the banding pattern because Fink-Heimer preparations of
the cortex after single-layer geniculate lesions show many coarse preterminal fibers
running tangentially in layer 4C, and more
of these fibers remain within single columns than cross columnar borders. This
segregation might give rise to the pale
bands if the coarser preterminal fibers began to thin out a short distance from the
true border. After lesions involving two adjacent geniculate layers the granular deposit, supposed to represent degenerating
boutons, forms a continuous band in the
fourth layer without any interruptions
(Hubel and Wiesel, '72). This indicates that
the finest preterminal arborizations and
the boutons themselves, belonging to two
adjacent columns, are either immediately
contiguous or overlap at the border. 1%nally, the physiology gives no suggestion of
a no-man's-land between neighboring columns - the recordings indeed show an
overlap of about 25-50 p , which may be
real, but is probably produced by the use of
electrodes whose uninsulated tip lengths
are themselves 25-50 p,
As was already indicated in the laminarlesion study, the width of the columns does
not vary with eccentricity but remains remarkably constant at about 300 p . When
the available space for stripes becomes reduced, as seems to occur during the streaming inward from the 17-18 border, there is
no reduction in stripe width; instead the
occasional stripe simply ends blindly while
its neighbors to either side coalesce. Hubel
and Wiesel ('74a) have found evidence for
a similar constancy and independence of
eccentricity in the width of columns subserving preferred stimulus orientation. The
total width of a set of columns covering
all 180" of orientation was estimated to be
0.5-1 mm, a value similar to the width of a
set of ocular dominance columns (one leftright pair). The significance of this uniformity in cortical function has been discussed (Hubel and Wiesel, '74b).
It is of considerable interest that the
overall pattern formed by the ocular domi-
572
SIMON LEVAY. D A V I D H. H U B E L A N D TOKSTEN N . W I E S E L
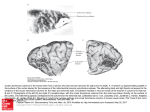
Fig. 8 Reconstructions of t h e ocular d o m i n a n c e columiis for ( a ) t h c ~o p c ~ c u l u m i, b ) roo! of thv c;ilc;irine fissure
a n d ( c ) t h e Ir;ives ,joining t h e roof t o thv stem. T h e midlincs is t o thr left. a n d the l u n a t e s u l c u s t o the top of the
d i a g r a m s ( c o m p a r e fig. 7). Dashed line: 17-18 border. T h e dotted lines represent t h e creases w h e r e t h e cortex i s
folded back on itsctlfor ( i n t h e middle of fig. 8 c ) t u r n s o u t of t h e planc o f t h e section to form t h e stem of t h e fissure.
T h e rcconstructions generally stop short of t h e dotted lines, hccariw th(, c u r v a t u r e becomes too great for t h e h a n d s
to be visible. B a r = 5 mm.
i-
8
C
574
S I M O N L L V A Y . DAVII) I 1
FIUBEL A N D TORSTEN N
nance columns was similar in all the animals examined, the individual brains differing only in the minute details of the
branching arrangements. The most orderly
part o f t h e pattern was at the 17-18 border,
which the columns always met more or less
at right angles. This arrangement may be
the most suitable one for the formation of
connections from area 17 to area 18 in the
immediate vicinity of the border, for it
implies that cells in left and right eye columns, serving the same point in the visual
field, are situated the same distance from
the 17-18 border. This would not have
been so if the columns had been arranged
parallel to the border.
Within area 17 the pattern formed by
the columns fell into two parts. Over the
smooth outer surface of the cortex, and extending a short distance onto the roof of
the calcarine fissure, the columns ran along
curves corresponding roughly to the representation of horizontal lines in the visual
field. Of possible relevance to this is the observation that the fourth layer of the striate cortex carries a double representation
of the visual field, one for the left eye columns and one for the right (Hubel et al.,
'74). This doubling of the field representation necessitates a corresponding halving
of the magnification factor in a direction
orthogonal to the columns, while the magnification along the columns remains the
same as the overall magnification. We have
been able to detect this anisotropy physiologically. The disposition of the columns
along the representation of horizontal lines
therefore results in horizontal components
of images in the central part of the field
being favored with a greater cortical magnification in the fourth layer than are vertical components. This arrangement could
conceivably be useful for stereoscopic vision,
since stereoscopy requires the detection of
small horizontal disparities between images in the two monocular representations.
Insofar as the relationship between column
direction and the representation of horizontal lines is not completely rigid - in particular, the area of foveal representation
has several columns running transversely
across the horizontal meridian - it is hard
to be sure whether the relationship has a
real significance or not. I t would be useful
in this connection to examine the cortex of
WIESk.1
different species, to see which features of
the pattern, if any, are shared by them.
More peripherally, in the calcarine fissure, the columns were oriented parasagittally, that is, roughly along the representation of circumferential lines in the visual
field. This may be the simplest arrangement
of the columns in the peripheral area, since
it means that if the cortex could be unfolded the columns would run directly from the
17-18 border on one bank of the stem of the
fissure to the 17-18 border on the other
bank.
There are two areas in the calcarine fissure which should be free of columns. One
is the monocular segment, which lies in the
lateral part of the stem of the fissure, mostly on the superior bank. This has been demonstrated autoradiographically (Wiesel et
al., '74), but it was not sectioned tangentially in the present study, and thus could
not be mapped. The other area is the representation of the optic disc. This is probably situated at the lateral end of the roof
o f t h e fissure, but we have not been able to
locate it precisely.
IIow is the columnar pattern arrived at
during development? The segregation of the
two sets of afferents into alternating bands
might be understood as the consequence of
two conflicting tendencies, the specification
of the two sets to occupy the same cortical
space according to a single topographic
map, and an opposing tendency for grouping within each set, as if caused by mutual
repulsion between members of the two sets.
The first principle would tend to increase
the degree of interpenetration of the two
sets, that is, to reduce the maximum distance from any cortical point to the nearest
column border. If this distance (which is
about 150 p) is kept constant, then a repulsion between the two sets would tend to
produce an arrangement with the smallest
total length of interface. Alternating bands
give a shorter interface than any other arrangement such as a checkerboard or circular islands in a matrix. But the constancy
of the overall pattern from animal to animal suggests that the afferents are also
guided by wide-field positional information.
One is tempted to think that the 17-18
border may be of importance in organizing
the pattern, because it is here that the pattern is most orderly. The developing cortex
.
TOPOGRAPHY OF OCULAR DOMINANCE COLUMNS
is rather inaccessible to experimental intervention, but the means by which the pattern is formed may become clearer from
studies of visually deprived animals. The
simple method of demonstrating ocular
dominance columns which has been described here promises to be useful in such
studies.
ACKNOWLEDGMENTS
We wish to thank Sarah Kennedy for histological assistance and Claire Wang for
help with the diagrams. This work is supported by the Rowland Foundation, Inc.,
the Esther A. and Joseph Klingenstein
Fund, Inc., and by NIH grants 5 ROI EYO
0605 and 5 ROI EYO 0606.
LITERATURE CITED
Daniel, P. M., and D. Whitteridge 1961 T h e representation of the visual field on the cerebral
cortex in monkeys. J. Physiol., 159: 203-222.
Hubel, D. H., and T. N. Wiesel 1965 Binocular
interaction in striate cortex of kittens reared with
artificial squint. J. Neurophysiol., 2 8 : 1041-1059.
575
1972 Laminar and columnar distribution
of geniculo-cortical fibers in the macaque monkey. J. Comp. Neur., 1 4 6 : 4 2 1 4 5 0 .
1974a Sequence regularity and geometry of orientation columns in the monkey striate cortex. J. Comp. Neur., 1 5 8 : 267-294.
1974b Uniformity of monkey striate cortex: a parallel relationship between field size,
scatter, and magnification factor. J . Comp.
Neur., 158: 295-306.
Hubel, D. H., T. N. Wiesel and S . LeVay 1974
Visual-field representation in layer IVC of monkey striate cortex. SOC. Neurosci.. 4th A n n u a l
Meeting (abstracts).
Jones, R. M. 1950 McClung’s Handbook of Microscopical Technique. Hafner, New York.
Lund, J . S. 1973 Organization of neurons in the
visual cortex, area 17, of the monkey (Mtrctrccr
mztldtcr). J . Comp. Neur., 1 4 7 : 4 5 5 4 9 6 .
Talbot, S . A,, and W. H. Marshall 1941 Physiological studies on neural mechanisms of visual
localization and discrimination. Amer. J . Ophthalmol.. 2 4 ; 1255-1246.
Wiesel, T. N., D. H. Hubel and D. Lam 1974 Autoradiographic demonstration of ocular domin a n c e columns in the monkey striate cortex by
means of transsynaptic transport. Brain Res..
79: 273-279.