* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Reductive Couplings
Bottromycin wikipedia , lookup
Elias James Corey wikipedia , lookup
Asymmetric hydrogenation wikipedia , lookup
Kinetic resolution wikipedia , lookup
Ene reaction wikipedia , lookup
Vinylcyclopropane rearrangement wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Organosulfur compounds wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Petasis reaction wikipedia , lookup
Asymmetric induction wikipedia , lookup
Hydroformylation wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
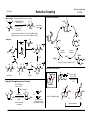
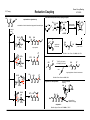
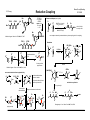
I.S. Young Baran Group Meeting 3/11/2009 Reductive Coupling Searching reductive coupling on Scifinder will lead to two major classes of reactions: 1) Alkyne to carbonyl compounds (aldehydes, imines and ketones): carbonyls lead to allylic alcohols. 1) Coupling of two carbonyl-type species to form pinacol type products - why is this good? no need for multistep functionalization to form an organometallic species, also more ammenable to asymmetric transformations. Example: McMurry coupling in Nicolau's synthesis of taxol O O Classes of Reductive Coupling HO OH OBn OBn R1 TiCl3 (DME)1-5, O H O O H O O O O Ti R1 H TMS alkyne used in many cases H LnNi H quench with electrophile yields 47-90% rr 86:24 to >96:4 use H2O for reductive OH R3 R4 + R2 R1 R3 R4 R2 OH Sato Tetrahedron Lett. 1995, 36, 3203 R3 R1 1) Ti(O-i-Pr)4 / i-PrMgCl R2 R1 H 2) N R2 R3 R5 R2 R5 R4 R3 R4 R1 + NH NH R3 R4 R2 yields 48-94% rr >20:1 in most cases then H2O reductive elimination R5 R1 R4ZnO - many factors influence the pathway taken such as; ligand, reductant, solvent R1 R4 R4 R1 key catalytic cycle intermediate OH R3 - addition of two molecules of aldehyde to titanocyclopropane not observed hydrogen introduction onto metal either from β-hydride elimination from ligand/reducing agent or introduced H2 gas R2 direct reductive elimination R2 R3 2) Transition metal catalyzed C-C bond forming event where a hydrogen (reductive coupling) is transferred instead of an alkyl group (alkylative coupling) in the reductive elimination step R4ZnO R1 behaves as a vincinal dianion equivalent Other C=X (X = heteroatom, ex. nitrones, oximes) etc can be used. SmI2 is frequently employed in the literature to induce this transformation R3 O O O LnNi O-i-Pr O Nicolaou Nature 1994, 367, 630 R4 O-i-Pr then R2 R2 Zn-Cu, DME 70 oC, 23% (O-i-Pr)2 Ti O R1 Ti(O-i-Pr)4 i-PrMgCl Sato Tetrahedron Lett. 1995, 36, 5913 Use of Ni to induce RC. ZnEt2 is the reducing agent so run risk of alkylative coupling (Et transfer) R4 OH R3 R2 alkylative coupling product R1 H H H R2 reductive elimination product R1 O R3 H X ZnEt2 R1 HO yields 62-74% Ni(COD)2 : PBu3 1:4 X Montgomery J. Am. Chem. Soc. 1997, 119, 9065 I.S. Young Mechanism R2 LnNi(0) O O L = PBu3 R1 H H O OH aigialomycin D L ZnR42 R2 CH3 MOMO OTBS OH Et3SiH (5.0 equiv) Ni(COD)2, IMes HCl t-BuOK (25 mol% each) use of NHC carbene ligand R3 R4ZnO R2 R1 H LnNi O O HO small substituent (or tether chain) R3 MOM O CH3 O R1 R2 H O large substituent R4 LnNi R4ZnO R3 Ni H H OH L L R3 R1 Baran Group Meeting 3/11/2009 Reductive Coupling L Me Ni R3 O R1 H Me N Cl N Me Me R2 MOM O O CH3 Me O Me IMes HCl MOMO L = THF OH R1 H H OH R3 R1 H R2 R4 R2 (intramolecular variant only) Reductive coupling would only occur on intramolecular R3 substrates with phosphine ligands - inter always led to alkylative with these coditions CH3 1) Et3SiH, Ni(COD)2 PBu3, THF 95% 2) HF pyridine 92% N H 3) Lio, NH3, THF 88% O H H3C OBn R1 Et3SiH also eliminates alkylative coupling Me OTBS First Catalytic in Nickel, also first intermolecular example using Ni O diethyl zinc adds to carbonyls in comples substrates 61% 1:1 dr Montgomery Org. Lett. 2008, 10, 811 Applications to Natural Product Synthesis Me OTES - terminal alkynes known to give poor dr as observed, but reaction of internal alkynes did not yield desired product R2 R3 H Me Ni(COD)2 (10 mol%) Bu3P (20 mol%) Et3B (200 mol%) R1 R3 R2 toluene or THF Jamison Org. Lett. 2000, 2, 4221 N OH yield = 45-89% rr = 92:8 to 98:2 Asymmetric Variants By Jamison H Me OH OH allopumiliotoxin 267A - cyclization step assembles six-membered ring, controls the relative stereochemistry adjacent to a quaternary center and assembles the alkylidene unit (each event occuring in a highly selective manner Montgomery J. Am. Chem. Soc. 1999, 121, 6098 .. P Me Ph Me Fe up to 67% ee J. Org. Chem. 2003, 68, 156 Me Me PPh2 (+)-NMDPP, up to 96% ee J. Am. Chem. Soc. 2003, 125, 3442 I.S. Young Applications to natural product synthesis H Me O Me Ph H + OTBS OH terpestacin Me alkyne/aldehyde coupling H .. O Me Me could envision forming the macrocycle by this method but wrong regioisomer was the only formed product (14 membered ring) Me 2 Ph Rh(COD)2OTf (5 mol%) R (R)-Cl-MeO-BIPHEP (5 mol%) 1 R2 DCE (0.1 M), 25 oC H2 (1 atm) Ph O Ph Ph D Ph Ph D Me Me HO O Bryostatin 1 R2 C7H15 Me O O D2 O RhIII(D)2Ln O OTBS Me O O O Me Rh(COD)2OTF (5 mol%) (R)-Tol-BINAP (5 mol%) Ph3CO2H (1.5 mol%) H2 (1 atm), ClCH2CH2Cl) 65 oC OH CO2Me OH 1,3-diynes and glyoxals: J. Am. Chem. Soc. 2003, 125, 11488 1,3-enynes and glyoxals: J. Am. Chem. Soc. 2004, 126, 4664 conjugated alkynes and ethyl (N-sulfinyl)iminoacetates: J. Am. Chem. Soc. 2005, 127, 11269 conjugated alkynes and α-ketoesters: J. Am. Chem. Soc. 2006, 128, 718 silyl substituted diynes to control regioselectivity: Org. Lett. 2006, 8, 3873 intramolecular acetylenic aldehydes cyclizations: J. Am. Chem. Soc. 2006, 128, 10674 heteroaldehydes and chiral Bronstead acid additived: J. Am. Chem. Soc. 2006, 128, 16448 in situ generation of enynes and coupling to carbonyls: J. Am. Chem. Soc. 2006, 128, 16061 in situ generation of enynes and coupling to imines: J. Am. Chem. Soc. 2007, 129, 7242 conjugated alkynes and α-ketoesters: Org. Lett. 2007, 9, 3745 application to the synthesis of hexoses: Org. Lett. 2008, 10, 4133 RhILn BnO OH O c Me O OAc OH Me O Ph MeO2C O O OH regioselectivity = 2.6:1 diastereoselectivity = 2:1 41% yield desired compound These rhodium procedures requires the alkyne to be conjugated for increased reactivity (Krishce Work, uses hydrogen as the reductant) O Ph OTBS Me O Ph D 81% H ligand O Ph OH Me Ph Fe OSiMe3 Ph LnRhID Ni(cod)2 (20 mol%) ligand (40 mol%) Et3B (150 mol%) ethyl acetate Jamison J. Am. Chem. Soc. 2003, 125, 11514 P Ph D Ph Me H Me O RhILn O R1 O Ph Ph Me Me Me catalytic cycle OSiMe3 O HO HO Baran Group Meeting 3/11/2009 Reductive Coupling BnO Me bryostatin C-Ring BnO OMe O Me c 4-steps Me O C7H15 O Me HO O CO2Me Krische Org. Lett. 2006, 8, 891 OTBS I.S. Young Reaction of alkynes with epoxides - first example of coupling to sp3 center (yields homoallylic alcohols) R1 Baran Group Meeting 3/11/2009 Reductive Coupling R2 + cat. Ni(cod)2 Bu3P R3 O R3 R1 R2 Et3B enatiomerically pure TBSO Me + Me O Ph Ni(cod)2 (10 mol%) Bu3P (20 mol%) Et3B >99% ee yield up to 85% >99% ee OH Me TBSO Ph O X cat. Ni(cod)2 Bu3P OH Ph yields up to 88% > 20:1 endo closure Et3B n n Me Ph benzylidene group is ozonized to produce the carbonyl group found in the natural product X OH 81% yield 99% dr Jamison J. Am. Chem. Soc. 2003, 125, 8076 Mechanism - endo opening suggests a different reaction mechanism than alkyne/aldehyde R R H PBu3 PBu3 Ni O O LnNi Ni R PBu3 OX R alkyne/ epoxide coupling OX R Me H Me O (5 steps) Me Ni(COD)2 (20 mol%) PMe2Ph (40 mol%) Et3B (150 mol%) toluene, 65 oC 82% yield n-Bu Me OH Ni(cod)2 (20 mol%) Bu3P (40 mol%) Et3B Me Me Ph Me CH2 HO O Jamison alkyne/ O aldehyde macrolactonization Me O Me pumiliotoxin 251D Jamison J. Org. Chem. 2007, 72, 7451 Me HO N H Ph Me Et3B Applications to Natural Product Synthesis n-Bu O PBu3 β−hydride Et Ni elimination OX O Me X= BEt2 N Me O Me Ph reductive elimination H Ni PBu3 R O O H anti-Bredt olefin accomadated by longer Ni-O and Ni-C bonds H steps Amphidinolide T1 O Me O Ph Me Jamison J. Am. Chem. Soc. 2004, 126, 998 O O 44% yield >10:1 dr I.S. Young Example to Show Fine Balance Between Alkylative and Reductive Coupling Development of NHC Ligands (allow for efficient intermoleuclar RC with triethylsilanes) - desired reaction is three-component alkylative coupling (RC was a competing problem) - imines less electrophilic - need hydroxylic solvent and organoboron reagent - methanol occupies coordination site, hindering β-hydride elimination R1 R2 H H Ni(COD)2 (10 mol%) (c-C5H9)3P (20 mol%) R5BX2 R4 HN R1 R4 R3 MeOAc/MeOH 0 oC, 20 h N R5 HN + R1 O alkylative coupling Ni PR3 Me H Me reductive coupling N + L Ar BEt3 Ar Me N BEt2 H Me Me O Ph H Mes N .. yield = 56-84% rr = >98:2 R3 N Mes R2 + Et3SiD Ni(COD)2 + Pr3SiH ligand X R3Si O Ph X From NHC From PBu3 Et Et Pr Pr H D H D <2 55 41 <2 25 34 23 18 using alkene as a regioselective director R3 R R2 Ar PR3 Me R1 H PR3 Me Ni N BEt2 Me H PR3 Me Ni N BEt2 Me aldehyde (n=0) or epoxide (n=1) Et3B R3 n R2 R1 Ni(cod)2/Cyp3P (cat) reference alkynes (not alkene-directed) alkene-directed Me Ar Me Ar R R4 OH effect of alkenyl group reverses regioselectivity N BEt2 Me Ar Ar reductive elimination Me Ni Me MeOH R1 R Me Me R3P OH Me Et Ni N BEt2 Me Et3SiH H Montgomery J. Am. Chem. Soc. 2004, 126, 3698 Me reductive elimination Et3Si O + R2 R2 Mechanism demonstrating competing pathways Me Ni(COD)2 (10 mol%) Cross Over Experiment to Show that Ligand Identity Affects Mechanism reductive coupling alkylative coupling (undesired) (major product with MeOH) yields 30-98% rr = > 10:1 Jamison Angew. Chem. Int. Ed. 2003, 42, 1364 alk:red = > 10:1 Me N BEt2 R1 R4 R3 R2 R3 + H R3 Et Baran Group Meeting 3/11/2009 Reductive Coupling 1 >20 >20 1 t-Bu t-Bu (does not couple) increases reactivity and controls regioselectivity 1 >20 Me circumvents poor regioselectivity β-H elimination 2 1 1 >20 >20 1 Jamison J. Am. Chem. Soc. 2004, 126, 4130 I.S. Young Baran Group Meeting 3/11/2009 Reductive Coupling directing effect of remote alkene and impact of phosphine application of synthetic approach: reaction doesn't work with 1,2,4 carbon spacer R1 OMs + R2 Et2BO Ni(COD)2 R1 O L with Cyp3P Ni R1 R Me O Me H Me R2 R or H OBEt2 without Cyp3P R1 R1 Pd(PPh3)4 Et2Zn C Me R1 O OH Me TiCl4 diaseteroselective propargylation (yields 60-82%) (d.r. 5:1 - >20:1) Me Me i) n-BuLi ii) ClTi(Oi-Pr)3, C5H9MgCl iii)BF3 OEt2, then Cyp3P Et3B R2 BEt2 Ni Ni H Me Et3B R2 CHO - two steps - two C-C bonds formed - three new stereocenters (*) - stereodefined trisubstituted double bond (*) - no protecting group manipulations O R2 R1 R1 O R2 Me Me Me Me R1 Me " " Me OH R2 * * * Me Me Me Me OH Me R2 H Me 15 Me O O Me Me MeO substrate for polyols (hydration/dihydroxylation), epoxides (olefin epoxidation and 1,5-diols (hydrogenation) Micalizio J. Am. Chem. Soc. 2005, 127, 3694. Me Me Me Me Me O HO NMe2 OR OMe O O H 1 Me MeO OH O ene-1,5-diol very valuable 9 Me O H Me Me Me HO two step general strategy: alkynation followed by alkyne/aldehyde reductive coupling R1 OH * O Me Jamison J. Am. Chem. Soc. 2004, 126, 4130 OH R1 O applications to total synthesis - degree of regioselectivity influenced by remote alkene - sense of regioselectivity controled by additive - with directing alkene and ligand combined, completely different mechanism OH regioselective reductive coupling yields 42-71% r.s. 3:1 - 19:1 d.s. 1.5:1 to 4:1 O H R2-CHO * * SiMe3 Me O Me NH MeO O R = CONH2 Me O macbecin 1 erythromycin A synthesis of the C-1 to C-15 fragment Micalizio Org. Lett. 2006, 8, 1181 Total Synthesis Micalizio Angew. Chem. Int. Ed. 2008, 47, 4005 I.S. Young Catalytic Version Mechanism Iridium Chemistry - non-conjugated alkynes can now be coupled R1 R2 O R3 OR4 O Ir(COD)2BARF (2 mol%) DPPF (2 mol%) Ph3CCO2H (2 mol%) H2 (1 atm) PhCH3, 60 oC O R2 R1 OR4 R3 OH yields: 73-99% rr usually > 20:1 Et Et Ph OBn O O Et O Et D2 Et OEt OH 94%, 95% D Et Me Mori J. Am. Chem. Soc. 1994, 116, 9771 OEt OH O2CR D H O DO2CR H Coupling of Other π-Components to Carbonyls OH N 100 mol% Ni(acac)2 200 mol% PPh3 Me N R1 n Ni complex OBn 1.5 eq Et3SiH toluene (also works catalytic) yield 52-86% 20 mol% Ni(cod)2 40 mo% PPh3 Et3SiH (5 equiv) THF CHO O elaeokanin C DIBAL-H (2 eq to Ni) O OBn Applications to total synthesis LnIrIII OH OSiEt3 Ni(0) Et3SiH O Et OEt LnIrIII Et O reductive elimination oxidative elimination O HO2CR Et OBn OEt LnIrIII IrIIILn Me Et3Si Ni H Et LnIrI O Ni SiEt3 OBn O Et Et Et D H insertion OEt IrIln Ni H O O Mechanism H Et3Si Alkynes and ketones: Krische J. Am. Chem. Soc. 2007, 129, 280. Alkynes and imines: Krische J. Am. Chem. Soc. 2007, 129, 8432. Et Baran Group Meeting 3/11/2009 Reductive Coupling R1 H Me OH R3 R3 = or n >2:1 ratio of double bond position isomers (internal usually favoured) H + O OSiEt3 N N Mori Tet. Lett. 1997, 38, 3931 OBn OSiEt3 37% O Mitsunobu Reaction 81% (4 steps) 36% I.S. Young CO2Me silylethylene-titanium alkoxide complex - reagent originally developed by Kulinkovich. Addition to an ester makes cyclopropanols. Utility expanded by Sato. HO O Baran Group Meeting 3/11/2009 Reductive Coupling H CO2Me 100 mol% Ni-complex 1,3-CHD (150 mol%) THF, rt O O Me Me O 53% (15% rcvd sm) O O Me Me R H 1,3-CHD is important to obtain double bond in the desired position. If it is not included it it migrates one position closer to the newly formed C-C bond. CO2H HO OH Me3Si OH 1) 10% Cp2Ti(PMe3)2 silyl hydride X Me NHR1 SiMe2 OEt CH3 X 2) workup CH3 X R2 Me3Si OEt Sato J. Org. Chem. 2000, 65, 6217 Me + H+ coupling of allenes / alkynes with chiral imines - reverse order, Ti reagent is complexed to imine instead of alkyne/allene OMe R HN major (from more stable metallocycle) Mechanism Cp2Ti(PMe3)2 Ph2(H)SiO H CH3 H3C +2PMe3 X N Ph 2 i-PrMgCl H CH3 X H R H yield = 28-84% d.r. >98:2 Ph Ti(O-i-Pr)2 OMe Ph R2 X H Ph2(H)SiO TiCp2 Ti(O-i-Pr)4 N Ar O H MeO Ph CH3 Cp2Ti CH3 H3C X -2PMe3 H+ HO OMe O Me X H Ar Buchwald J. Am. Chem. Soc. 1995, 117, 6785 Crowe J. Am. Chem. Soc. 1995, 117, 6787 48% SiMe2 75% OH Me R Me3Si H+ Ti(O-i-Pr)2 ketones and alkenes - stated as the first catalytic for alkene/alkyne with heteroatom containing DB. Mori did it with dienes and aldehydes in 1994...? O 34-87% R1 R2 Total Synthesis by Mori, Synlett 1997, 734 PGF2α Me3Si OH N HO H+ R1 X - chiral group can be removed to produce chiral primary amines Sato Org. Lett. 2003, 5, 2145 HN Ar Ph C H R2 R1 yields = 45-74% d.r. >98:2 I.S. Young Baran Group Meeting 3/11/2009 Reductive Coupling Allenes and Transfer hydrogenation: homo-allylic alcohols and imines - more complex RC as you generate two new stereocenters. OH R1 R3 + Ti(O-i-Pr)4 c-C5H9MgCl N HN yields 61-83% r.r. > 20:1 d.r. = 4:1 to >20:1 R2 H OH (i-PrO)n R1 R1 R3 PPh3 / CCl4 R1 reflux C R2 cyclization R2 N 76-85% R3 R3 O Ti N H R2 Micalizio J. Am. Chem. Soc. 2007, 129, 7514 C R Me aryl or activate alkyl aldehyde - in many cases 4 to 8 equivalents of allyl source is required (no mention of this!) R1 OH C OH R2 R3 R Li2CO3 (35 mol%) DCE-EtOAc (1:1), 60 oC H2 (1 atm) Me Me O yields: 60-95% Me Standard Conditions D2 (1 atm) R1 R2 yields: 23-92% - can also use rhodium based catalysts [RuHCl(CO)(PPh3)] to affect similar transformations but an acid cocatalyst (m-NO2BzOH or CF3CO2H) is required when the alcohol is used as the substrate. OH Other sources of allyl derivative metal reagents for transfer hydrogenation O NO2 R3 Cs2CO3 (5-7.5 mol%) DCE-EtOAc (1:1), 75 oC No H2 Krische J. Am. Chem. Soc. 2007, 129, 15134. D C OH [Ir(BIPHEP)(cod)]BARF (5-7.5 mol%) - alcohol serves as hydrogen source and electrophilic substrate - eliminates the need for oxidation prior to allyl nucleophile addition Mechanism - prenyl group is necessary to prevent over reduction of double bond O yields: 50-90% - basic additive Cs2CO3 also helps to inhibit over-reduction Krische J. Am. Chem. Soc. 2007, 129, 12678 Me R1 R2 - no stoichiometric by products produced R1 [Ir(BIPHEP)(cod)]BARF (5 mol%) O R3 Cs2CO3 (5-7.5 mol%) DCE-EtOAc (1:1), 75 oC i-PrOH (200 - 400 mol%) - transfer hydrogenation must be used since H2 over reduces all products besides reverse prenyl - reverse prenylation with allenyl metal reagents Me R3 R2 OH [Ir(BIPHEP)(cod)]BARF (5-7.5 mol%) O Me NO2 Me R1 R2 LnIr-D LnIr-D D2 D D D LnIr Me Me LnIr O OH Me R Me O Me Me NO2 OAc OAc 1,3-cyclohexadiene Org. Lett. 2008, 10, 1033 allyl acetate JACS 2008, 130, 6340 asymmetric JACS 2008, 130, 14891 acyclic dienes JACS 2008, 130, 6338 Me asymmetric crotylation JACS 2009, 131, 2415 I.S. Young Baran Group Meeting 3/11/2009 Reductive Coupling Vinyl pyridines and imines ArSO2 R1 Allene Alkyne Coupling [Rh(cod)2]BARF (5 mol%) (2-Fur)3P (12 mol%) N N R1 R2 R2 N Na2SO4 (200 mol%), 65 oC DCM, 25 oC H2 (1 atm) R2 H NSO2Ar R1 C Me R2 1) Ti(O-i-Pr)2 + R3 56-97% yield 3:1 -13:1 dr R1 Sato Chem. Commun. 1998, 271. Effect of alkoxide on stereochemistry π-π coulpings where the π-bonds are all carbon C6H13 1) i-PrMgCl Paper was about alkylative cyclization but they found that use of phosphine led to RC alkylative vs reductive cyclizaiton if X = H H+ Bu2Zn / BuZnCl 2) H Ni(COD)2, 5 mol% Ph PPh3, 25 mol% BuZnO X = TBS 62:38 H 82:18 reduction or alkylation 1) i-PrMgCl if X = H H+ without phosphine Bu (51%) R2 = H (11%) with phosphine R2 = H (92%) R2 R1 + Me XO XO 2) R2 = 63% 69% SiMe3 SiMe3 XO NiLn Me Me Ti(O-i-Pr)2 X = TBS H SiMe3 H H + XO XO R2 O C6H13 C6H13 XO O H R3 2) H+ - need substitution at 6-position of pyridine, otherwise catalyst binds to nitrogen and no reaction Krische, J. Am. Chem. Soc. 2008,130, 12592 Ph yields 45-94% E:Z ration 64:36 to >20:1 Me Ti(O-i-Pr)2 X = TBS H X = TBS 51:49 H 90:10 73% 70% - result explained by the fact that the alkoxide is better at locking the transition state in a chair than the -OTBS even though it is "smaller" phosphine may force alkyl and alkenyl into a trans orientation, thus preventing reductive elimination Sato Tetrahedron Lett. 1998, 39, 7329 Montgomery J. Am. Chem. Soc. 1996, 118, 2099 Montgomery J. Am. Chem. Soc. 1997, 119, 4911 alkyne-alkyne coupling to prepare 1,3-dienes Allenyne Cyclizations R3 Ti(O-i-Pr)2 R1 R2 n C R1 R3 R4 R1 R2 R1 R1 + yield = 42-98% rr 4:1 to >20:1 n R3 Ti(O-i-Pr)2 R2 R2 R1 Ti(O-i-Pr)2 R2 R3 yields 47-93% rr = 60:40 to >20:1 R4 Sato J. Am. Chem. Soc. 1997, 119, 11295 R2 Sato J. Am. Chem. Soc. 1999, 121, 7342 R3 I.S. Young Baran Group Meeting 3/11/2009 Reductive Coupling Cobalt Becomes Involved - alkyne / activated alkene coupling Proposed mechanism shown for diyne: Ph R1 R2 + R3 R3 = acceptor [CoI2(PPh3)2], PPh3, Zn R3 R1 CH3CN, H2O, 80 oC R2 MeO2C Ph MeO2C Ph MeO2C RhIIILnD MeO2C Ph mechanism Ph CoII Zn Me Co CO2-nBu CoI LnRhIOTf D2 CO2-nBu LnRhID H2O Ph CoIII CoIII MeO2C D MeO2C D CO2-nBu RhILn MeO2C D Ph MeO2C RhIII(D)2Ln MeO2C D D2 Ph Ph Me MeO2C Ph Ph Zn Ph CO2-nBu Ph Me Cheng J. Am. Chem. Soc. 2002, 124, 9696 Alkyne-Alkyne Coupling by Micalizio (Similar to Sato) previous Krische examples showed metal adding across C-O π bond, why not C-C π bond? R1 X R1 Rh(COD)2OTf (3 mol%) rac-BINAP or BIPHEP (3 mol%) R2 DCE (0.1 M), 25 oC H2 (1 atm) - Micalizio different for two reasons: functionalization of the internal alkyne component has only been achieved with TMS-substituted alkynes and conjugated 2-alkynoates (this introduces limits for polyketide synthesis) yields 51-90% X - coupling can lead to four possible regioisomers R2 R X DCE (0.1 M), 25 oC H2 (1 atm) R1O R Rh(COD)2OTf (3 mol%) rac-BINAP or BIPHEP (3 mol%) OR2 Me ClTi(Oi-Pr)3, c-C5H9MgCl -78 to -30 yields 65-91% X Me CH3 Me R1O -78 oC then terminal alkyne yields = 46-87% r.r. = 5:1 to 8:1 Micalizio Org. Lett. 2005, 7, 5111. diyne and enyne cyclizations: Krische J. Am. Chem. Soc. 2004, 126, 7875 asymmetric enyne cyclizations: Krische J. Am. Chem. Soc. 2005, 127, 6174 OR2 oC R3 Me Me Me I.S. Young R1O OH Me Baran Group Meeting 3/11/2009 Reductive Coupling alkoxide directed (intramolecular) coupling of alkynes Me explanation for regioselectivty minimization of steric interactions in approach of second alkyne Me i-Pr O Oi-Pr Ti R1 + Li O Ti catalyst then R2 n O Oi-Pr Ti n R2 R1 R2 R2 intramolecular carbometalation n R2 O Oi-Pr Ti R2 R1 R2 + LiOiPr R1 R2 i-Pr O O OH R2 R2 Ti RL Me Me Oi-Pr Me Me R2 HO regioisomer not formed major product R2 R1 R1 Oi-Pr R1O yields 51-58% n n R2 R2 H+ R2 OH Micalizio J. Am. Chem. Soc. 2006, 128, 2764 also works with allenes to make skipped 1,4-dienes OH Ti RL Me Me Oi-Pr Me R3 OH Me Ti(Oi-Pr)4 (2.1 equiv) c-C5H9MgCl (4.2 equiv) C R1 R2 R1O i-Pr O OH RL Me Me Me Me Application to total synthesis Oi-Pr palladium-mediated coupling Me Oi-Pr R2 R3 Micalizio Chem. Commun. 2007, 4531 R2 Ti R2 single regioisomer formed in most cases R4 R2 R5 R1 R5 then R4 OH R1O HO R2 OH Ti Me O H O O Me RL Me Oi-Pr Me Me Me Me Me Me Me alkyne-alkyne reductive coupling callystatin A Micalizio Angew. Chem. Int. Ed. 2008, 47, 7837. I.S. Young TBS O OTMS Me + Me Me Me Me H O Enones and alkynes (enals cyclize) ClTi(OiPr)3 c-C5H9MgCl toluene Me OiPr O Me Me OTMS H O R2 Me MLn M R3 H R2 R1 H R3 1) carbometallation R1 2) H+ R4 BEt2OMe R1 Et2BOMe + R2 R3 R4 R1 R4 R4 + Me R4 rr usually >20:1 Mechanism O Me yield = 50-90% R1 Et3B (3.0 equiv) MeOH, THF (8:1) R3 R2 O - can couple enoates with ynoates without any homocoupling, which is surprising OiPr Me Me Ni(COD)2 (10 mol%) PBu3 (20 mol%) + R2 Micalizio Angew. Chem. Int. Ed. 2008, 47, 7837. TBS O R4 R1 75% yield r.r. 5:1 Me R1 Baran Group Meeting 3/11/2009 Reductive Coupling O NiLn Ni(0)Ln OBEt2OMe Ni Ln or R4 R4 R3 R2 R1 R2 R2 R3 R3 R3 3 other possible regioisomers MeOH Micalizio Angew. Chem. Int. Ed. 2007, 46, 1440 (MeO)Et2 B O use remote alkoxide to direct regioselectivity Li O RE iPr O OiPr Ti + n RZ R2 R2 RE intramolecular carbometalation R2 OH R2 n RZ RE (major product) H+ O OiPr H Ti R2 or n RZ O OiPr Ti RE 2 R R2 R4 - without alkoxide there was no reaction RZ n OBEt2 Ln Ni R2 bridged conformation less favoured O R2 R2 O Ti OiPr RE n RZ R2 H+ H O R2 R3 O R4 R2 H NiLn product R1 R4 R3 RE n RZ R3 R2 = aryl or alkyl NiLn R3 R4 enal leads to cyclization Et R1 R2 R2 R2 R1 OH R4 =H R3 R1 - only works with allylic and homo allylic alcohols R1 Montgomery J. Am. Chem. Soc. 2007, 129, 8712 I.S. Young Cobalt mediated alkyne-alkene HO [CoI2(dppe)], ZnI2, Zn X n application to total synthesis R1 R1 CH3CN, H2O, 80 R2 + R3 [CoBr2(dppe)] Zn, ZnI2, CH2Cl2 Me Me alkyne/allylic alcohol reductive coupling OAc R2 OH phorbasin C no additional coupling even though product is again an allylic alcohol key step in total synthesis Me Me Co R2 Me n Cobalt mediated alkyne-unactivated alkene coupling R2 Me O X oC Cheng J. Am. Chem. Soc. 2007, 129, 12032 R1 Baran Group Meeting 3/11/2009 Reductive Coupling R3 β-hydrogen elimination R1 R2 Me R3 O Me Me O Ti(Oi-Pr)4 c-C5H9MgCl, Et2O + R1 -78 oC to rt HO Treutwein Angew. Chem. Int. Ed. 2007, 46, 8500 O TMS 47% dr > 20:1 HO TMS Me O OH alkynes and allyl alcohols with transfer of oxygen to catalyst (net allyl transposition) R4 ii) R5 R2 i) ClTi(Oi-Pr)3, c-C5H9MgCl Complimentary Claisen-based methods: a stereodivergent product is produced R4 R1 Micalizio J. Am. Chem. Soc. 2009, 131, 1392 R5 OH O Li R1 R3 R3 R1 yields 42-79% rr from 1:1 to >20:1 R2 i) Claisen rearrangement R3 R1 OH R2 ii) reduction R2 typically > 20:1 alkene substitution pattern has a large impact on degree of selectivity then H+ R3 intermediate This work: R1 (Oi-Pr)n Ti R1 Me H O R2 H+ Me Me workup R1 R2 major product by control of A-1,3-strain R1 Me Si Cl i) ClTi(Oi-Pr)3 c-C5H9MgCl ii) O R3 R1 R1 R3 Tamao R3 then 1N HCl t-BuOOH, CsOH, TBAF DMF [Si] Oxidation Li R2 Micalizio J. Am. Chem. Soc. 2007, 129, 15112 similar results reported by Cha J. Am. Chem. Soc. 2008, 130, 15997 R1 R2 typically > 20:1 OH R2 Micalizio J. Am. Chem. Soc. 2008, 130, 16870 Cha (J. Am. Chem. Soc. 2008, 130, 15997) reported this result first, but allylic alcohols were limited to cyclohexyl derived, except for one case I.S. Young Reductive Aldols - no prefunctionalization with stoichiometric reagents O R2 R3 + R1 Me3SiH RhCl3 3H2O O R4 O R4 R3 If = OMe, no evidence of silyl ketene formation and then aldol R5 R3 R3 R1 O R4 R2 R5 H Rh4(CO)12 (cat.) R1 R5 R5 R2 Matsuda Tetrahedron Lett. 1990, 31, 5331 R1 R4 R3 H + yields - 22-99% dr 55:45 to 84:16 1) 2.5 mol% [(COD)RhCl]2 5.5 mol% Me-DuPhos Cl2MeSiH O OR2 OH O R1 R1 H H 1) 2.5 mol% [(COD)RhCl]2 6.5 mol% R-BINAP Et2MeSiH 2) H3O+ 1) 2.5 mol% [(COD)IrCl]2 7.5 mol% ligand Et2MeSiH 2) H3 O OMe H OH R R2 yield 44-92% syn:anti 1.7 to 2.5:1 R 1 2 H2 (1 atm), KOAc (30 mol%) DCE, 25 oC For Ketone Additions to Aldehydes Acceptors:J. Am. Chem. Soc. 2002, 124, 15156 For For Ketone Additions to Ketone Acceptors: Org. Lett. 2003, 5, 1143 For Aldehyde Addition to Glyoxals Acceptors: J. Org. Chem. 2004, 69, 1380 For Aldehyde Additions to Ketones Acceptors: Org. Lett. 2004, 6, 691 Increase in syn selectivity using tri-2-furylphosphine: Org. Lett. 2006, 8, 519 Unsymmetrical divinyl ketone addition to aldehydes: Org. Lett. 2006, 8, 5657 Ketone addition to α-amino aldehydes (syn stereotriads): J. Am. Chem. Soc. 2006, 128, 17051 Asymmetric ketone additions to aldehydes: J. Am. Chem. Soc. 2008, 130, 2746. O O Ph - treatment of substrate with only phosphine does not lead to Baylis-Hillman OH O R1 O+ O Me OH R A H2 yields 48-72% syn:anti 1.8:1 to 5.1:1 2 OR ee (syn) = 45-88% O Ph Morken J. Am. Chem. Soc. 2000, 122, 4528 R R1 O Rh(COD)2OTf (10 mol%) (p-CF3Ph)3P (24 mol%) O LnRhIII(H)2 OR2 O O - The KOAc helps prevent conjugate reduction by deprotonating complex A or B Enantioselective Variants + n Mechanism Me Morken, J. Am. Chem. Soc. 1999, 121, 12202 O yield 64-90% syn:anti 5 to 20:1 OR2 2) H3O+ did experiements in 192 well plates to determine ideal conditions O OH R H2 (1 atm), KOAc (30 mol%) DCE, 25 oC n OSiMeEt2 O R2 O O R O Rh(COD)2OTf (10 mol%) (p-CF3Ph)3P (24 mol%) yields = 24-95% O + Et2MeSiH3 + Hydrogen as the reductant - All work by Krische OSiMe3 R3 Revis Tetrahedron Lett. 1987, 28, 4809 O Baran Group Meeting 3/11/2009 Reductive Coupling LnRhI avoid this Ln Me yields 47-68% syn:anti 1.7:1 to 9.1:1 ee (syn) = 82-96% Morken Org. Lett. 2001, 3, 1829 O O N N O N ligand Ph III O O LnRh conjugate reduction O OMe H O OH Ph H Ph B H RhIII O enolate addition I.S. Young Baran Group Meeting 3/11/2009 Reductive Coupling Role of aryl halides in Ni-Catalyzed Reductive Aldols Three-Component Coupling via Internal Redox (leads to esters and malonates!) O O + O-t-Bu H OH Et3B Ni(COD)2 R O R PhI O-t-Bu Three-Component enal, alkyne, alcohol additions (no reducting agent is necessary, it forces reaction to the cycloaddition pathway). No Reaction Without PhI CH3 O - PhI isn't just serving as a mechanism to generate Ni(II) from Ni(COD)2 R1 H - proposed to form a boron enolate which then reacts with aldehyde (metal no longer complexed for this step). R4 + MeOH, THF (8:1) Montgomery Org. Lett. 2007, 9, 537 N O + R1 R3 R4 R2 HO Ni(COD)2 (10 mol%) PBu3 (20 mol%) R2 R3 LNi(OMe)2 (doesn't re-enter cycle) H OMe R2 R2 Ph H O OMe R2 O H LnNi R1 MeO NiLn R2 R1 NiL R1 Three-Component Enone, Alkyne, Aldehyde Additions R1 Ni O R2 O R1 H H R1 O O ONiL H Ln Ni O MeOH R1 OMe L = Me2N NMe2 (1 equiv) Ph + LnNi(0) NiLn R4 Ph OH O R2 MeOH need to add co-reductant H R1 Ni(COD)2 (1 equiv) Ph N yield 58-85% good dr Original Stoichiometric Protocol (only worked for intramolecular) H O H Montgomery J. Am. Chem. Soc. 2006, 128, 14030 O R3 Cl Mechanism R5 R1 Et3B (4.0 equiv) MeOH, THF (8:1) R4 R1 Random Reactions R5 H O R3 R2 Intermolecular Enal-Alkyne [3+2] Reductive Cycloadditions (first example of catalytic and intermolecular) -synthesis of carbocyclic 5-membered rings via cycloaddtions difficult (traditional 3+2's need a heteroatom in dipole) - formal solutions is to use strained rings precursors, vinyl carbenoids or dianion equivalents - the assembly of an odd-membered ring from two even numbered pi systems would require a net two electron oxidation or reduction or a hydride shift - early stoichiometric work in the field by Sato J. Am. Chem. Soc. 1996, 118, 8729 and J. Am. Chem. Soc. 1997, 119, 10014 OMe R2 Ni(COD)2, KO-t-Bu O R2 + R3 R5 + H R4 Ni(COD)2, Ligand H R2 R5 O toluene Ph Montgomery J. Am. Chem. Soc. 2008, 130, 469 O R3 R4 I.S. Young Reductive Coupling Mechanism O R2 R1 R5 + R2 Ln Ni O LnNi(0) R1 RS R4 R1 H O O O NiLn H O Ln Ni R2 R5 R4 R3 H R5 R1 NiLn R2 R4 O O R1 R3 R5 R3 R2 R3 RL R4 H R2 R5 O O R1 R4 Reviews on Reductive Coupling Sato: Bicyclization of dienes, enynes, and diynes with Ti(II) reagetn. New developments towards asymmetric synthesis. Pure Appl. Chem. 1999, 71, 1511. Sato: Synthesis of organotitanium complexes from alkenes and alkynes and their synthetic applications. Chem. Rev. 2000, 100, 2835. Montgomery: Nickel-catalyzed cyclizations, couplings, and cycloadditions involving three reactive components. Acc. Chem. Res. 2000, 33, 467. Montgomery: Nickel-catalyzed reductive cyclizations and couplings. Angew. Chem. Int. Ed. 2004, 43, 3890. Cheng: Cobalt- and nickel-catalyzed regio- and stereoselective reductive couplings of alkynes, allenes, and alkenes with alkenes. Chem. Eur. J. 2008, 14, 10876. Krische: Catalytic carbonyl addition through transfer hydrogenation: A departure from preformed organometallic reagents. Angew. Chem. Int. Ed. 2009, 48, 34. Jamison: Nickel-catalyzed coupling reactions of alkenes. Pure Appl. Chem. 2008, 80, 929. Baran Group Meeting 3/11/2009