* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download MOVEMENT AND GUIDANCE OF MIGRATING MESODERMAL
Cytokinesis wikipedia , lookup
Cell growth wikipedia , lookup
Tissue engineering wikipedia , lookup
Cell encapsulation wikipedia , lookup
Cellular differentiation wikipedia , lookup
Cell culture wikipedia , lookup
Extracellular matrix wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
J. Cell Set. 56, 207-222 (1982)
2O7
Printed in Great Britain © Company of Biologists Limited 1982
MOVEMENT AND GUIDANCE OF
MIGRATING MESODERMAL CELLS IN
AMBYSTOMA MACULATUM GASTRULAE
NORIO NAKATSUJI, ANDREW C. GOULD
AND KURT E. JOHNSON
Department of Anatomy, The George Washington University Medical Center,
Washington, D.C. 20037, U.S.A.
SUMMARY
A scanning electron microscopic study in early gastrulae of Ambystoma maculattan showed
that migrating presumptive mesodermal cells were strongly oriented toward the animal pole.
They had lamellipodia and filopodia at their leading edges, and rounded or tapering, tail-like,
trailing edges. Of the cells whose polarization could be determined unequivocally, 81 %
appeared to be directed in a quadrant toward the animal pole, and 93 % were directed to some
extent away from the blastopore. This strong orientation suggests that specific mechanisms
direct cell movement, in addition to the non-specific dispersive mechanism of the contact
inhibition of cell movement. There is a network offineextracellular fibrils that covers the inner
surface of the ectodermal layer. Filopodia of the migrating cells frequently attach to and appear
to follow the fibrils, suggesting that the fibrils serve as a guiding substratum for cell attachment
and movement. There are areas where the fibrils are apparently aligned along the blastopore animal pole axis, and a preliminary statistical analysis using micrographs at high magnification showed a significant alignment parallel to the blastopore - animal pole axis. This fibril
alignment could cause the strong orientation of the mesodermal cells by means of contact
guidance.
INTRODUCTION
Gastrulation involves extensive morphogenetic movements where the direction of
movement of each cell group is somehow controlled, so that presumptive cells of each
tissue and organ are eventually distributed appropriately to form the three primary
germ layers. In the pioneering work on the mechanism of gastrulation in amphibian
embryos, Holtfreter (1943, 1944) proposed two forces as the causes of these specific
movements: imagination of the bottle cells and epibolic expansion of the ectodermal
layer. The latter force was recently studied by Keller (1978, 1980) using cinemicrography and scanning electron microscopy. He found that in Xenopus gastrulae,
expansion appears to be associated with a radial interdigitation of deep cells and a
spreading of superficial cells. Although the bottle cells are the most morphologically
remarkable cells in gastrulae, and thus have attracted a good deal of attention
(Rhumbler, 1902; Rumni, 1925; Holtfreter, 1943), more recent studies suggest that
bottle cells are not crucial for gastrulation. For example, gastrulation proceeds in
spite of the removal of the bottle cells (Cooke, 1975; Keller, 1981). Also, no cell
movement occurs, in spite of the presence of the bottle cells, when a hypertonic
sorbitol solution is injected into the blastocoel of Xenopus gastrulae (Nakatsuji, 1979).
2O8
N. Nakatsuji, A. C. Gould and K. E. Johnson
45'
135
45°
135°
Fig. i. Measurement of the angle (0) made by the orientation of the migrating mesodermal cell and the direction toward the animal pole (ap). bp, blastopore.
Since the first finding of pseudopodia formed by the migrating mesodermal cells
in Bufo gastrulae (Nakatsuji, 1974), filopodia, lamellipodia and blebs have been
observed on migrating mesodermal cells of various species of anurans and urodeles
(Nakatsuji, 1975, 1976; Keller & Schoenwolf, 1977; Kubota & Durston, 1978). Their
formation and attachment to the inner surface of the ectodermal layer suggest that
the mesodermal cells move actively using the ectodermal cells as their substratum.
This point is supported by a cinematographic study of the cell migration in bisected
urodele gastrulae (Kubota & Durston, 1978). The gastrula mesodermal cells show
active and rapid locomotion in vitro when dissociated and cultured in adequate conditions (Nakatsuji & Johnson, 1982). Thus, at present, we can identify two major forces
that appear to be essential in gastrulation: (1) the migration of the mesodermal cells
on the inner surface of the ectodermal layer, and (2) the epibolic expansion of the
ectodermal layer.
For the migrating mesodermal cells to move preferentially toward the animal pole,
one would predict that they should produce dominant locomotory organelles more
frequently in that direction. A strong orientation of lamellipodia toward the animal
pole is shown in this scanning electron microscopic study. The extracellular matrix
components have been suggested as candidates for control of the cell migration in
many cases such as that of neural crest cells (Lofberg, Ahlfors & Fallstrom, 1980).
Johnson (igjja-d, 1978) studied the synthesis and biochemical nature of the extracellular matrix materials in frog gastrulae, and showed that they promote gastrula
cell adhesion when coupled to CNBr-Sepharose beads (Johnson, 1981). However,
there have been no morphological observations showing the existence of a basal
lamina or other definite extracellular matrix structure in amphibian gastrula stage
embryos, though suggestive observations have been published (Johnson, Silver &
Kelley, 1979). In neurulae, Karfunkel (1977) has reported the existence of extracellular
Cell migration in Ambystoma gastrulae
209
Fig. 2. Diagrams showing the method of a statistical analysis of the orientation of the
fibrils. The left diagram shows superimposition of the traced fibrils on the graph paper.
The right diagram shows determination of the instantaneous slope (y/x) of the fibril
at the intersection with vertical and horizontal lines of the graph paper, ap, animal
pole.
fibrils on the inner surface of the ectodermal layer in the lateralflankregion. Ambystoma
gastrulae have an anastomosing network of extracellular fibrils covering the inner
surface of the ectodermal layer, the very substratum for the migrating mesodermal
cells. The filopodial extensions from migrating mesodermal cells often project along
extracellular fibrils as if they adhere to them preferentially.
MATERIALS AND METHODS
Embryos and scanning electron microscopy
Ambystoma maculatwn eggs were collected in small ponds near Chapel Hill, North Carolina,
or purchased from Connecticut Valley Biology Supply Co., Southampton, Massachusetts.The
jelly coats and vitelline membranes surrounding embryos were removed mechanically with fine
forceps. Early gastrula-stage (Harrison stage 10; Rugh, 1962) embryos were immersed in a
fixative solution of 2-5 % glutaraldehyde in o-i M-sodium cacodylate buffer (pH 7-2) and cut
from the ventral side with fine forceps and hair loops, exposing the inner surface of the dorsal
ectodermal layer with the attached migrating cells. We will call these cells the migrating mesodermal cells in this paper, although the presumptive endodermal cells of the head region are
probably present among them as well. The endodermal cell mass was left attached to the
vegetal end of the ectodermal cell layer to mark the vegetal side. Specimens were fixed for
1 day at room temperature in the same fixative solution, and then post-fixed with 1 % OsO« in
the same buffer for 1 h at room temperature. They were dehydrated through a graded ethanol
series, critical-point dried through liquid COt, and sputter-coated with gold/palladium at a
thickness of about 20 nm, and examined in a JEOL JSM-35 scanning electron microscope.
Determination of cell polarity
The polarity of migrating mesodermal cells in scanning electron micrographs was determined
by identifying a flattened end of the cell with one or more lamellipodia and a rounded or
tapering end. First, we made montages of scanning electron micrographs of four embryos, and
traced the outline of the migrating mesodermal cells. Arrows showing cell polarity were drawn
from the middle of the rounded or tapering end toward the middle of the flattened end with
lamellipodia. The angle (&) made by the arrow and the line parallel to the blastopore - animal
pole axis was measured as shown in Fig. i, and scored into one of four quadrants: toward the
210
N. Nakatsuji, A. C. Gould and K. E. Johnson
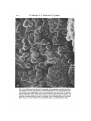
Fig. 3. A montage scanning electron micrograph of the migrating mesodermal cells on
the dorsal part of the inner surface of the ectodermal layer of an early gastrula. The
inset shows a low-magnification view. The animal pole is to the left of the picture,
and the blastopore to the right. Note that many cells are directed toward the left: the
flattened ends that attach to the inner surface of the ectodermal layer to the left, and
rounded or tapering ends that are not attached to the ectodermal layer to the right.
Bars, ioo /im. e, inner surface of the ectodermal layer; n, endodermal cell mass.
Cell migration in Ambystoma gastrulae
211
0
animal pole (0 < 45°), away from it (0 £ 135°). to the left or the right (45 g 0 < 135°).
Another way of scoring was either toward {6 < 900) or away from (0 ^ 900) the animal pole.
Orientation of the extracellular fibrils
Scanning electron micrographs at a magnification of 3000 x were assembled into montages
that show the extracellular fibrils on the inner surface of the ectodermal layer. The areas for
montages were selected by observing the specimens at a low magnification where fibrils were
not visible, and selecting flat open areas of the ectoderm surface just in front of the migrating
mesodermal cells. Five montages such as Fig. 7 were made from three embryos. They cover
the area of from 3800 /1m1 to 10000 fun* each, with a total of 32300 /an1. Each montage was
then covered with a clear acetate sheet. On each sheet, the paths of the underlyingfibrilswere
traced with a fine-point ink marker.
Next, these acetate overlays were superimposed on graph paper with the blastopore - animal
pole axis parallel to the vertical axis of the graph paper, as shown in Fig. 2. Using both vertical
and horizontal lines spaced 0-7 cm apart on the graph paper, every intersection with a fibril
was identified. The slope of a line tangent to the fibril at the point of intersection was then
determined (Fig. 2). The number of the intersections where the absolute value of the slope was
greater than or equal to unity (within 45° from the blastopore - animal pole axis) was divided
by the number of those with less than unity (within 45° from the perpendicular line to the blastopore - animal pole axis) to yield a ratio. This ratio (the alignment ratio) was used as an indicator of the alignment of the fibrils. A random distribution would yield an alignment ratio of
unity, while an alignment along the blastopore - animal pole axis would give a ratio greater
than unity, and an alignment perpendicular to it would give a ratio less than unity. The number
of the intersections where the absolute value of the slope is equal to unity was less than 10%
of all the intersections.
This method removes much of the investigator's bias in determining the degree of fibril
orientation, as it does not require determination of the slope of individual fibrils, which, in
most cases, are neither straight nor unambiguously distinguishable from other fibrils that may
cross or join them. The method also weights the significance of the orientation of afibrilaccording to its length, since the number of intersections of a fibril with the graph lines (and thus the
number of determinations of the slope) is proportional to its length. The close spacing of the
graph lines also ensures that nearly every fibril is counted at least once.
RESULTS
Cell shape and orientation of the migrating mesodermal cells
In early gastrulae, presumptive mesodermal cells have just begun to migrate toward the animal pole on the dorsal side of the embryo (Fig. 3, inset). The migrating
cells do not make a cohesive cell sheet, but seem to be migrating as an unorganized
group of cells on the inner surface of the ectodermal cell layer with large intercellular
spaces between them (Fig. 3) but not extensive overlapping. Extension of the archenteron, a cohesive cell sheet, follows them at later stages. The migrating mesodermal
cells have a rounded cell body and lamellipodia, from which fUopodia extend
(Fig. 4A, B). Some parts of the surface of these cells are relatively smooth while others
have blebs and microvilli (Fig. 5). The lamellipodia and filopodia are either attached
to the inner surface of the ectodermal layer, or sometimes to other migrating cells.
Many cells are stretched in one direction and have features that suggest oriented cell
movement, i.e. a dominant lamellipodium on one side of the cell, presumably representing a leading edge, and the opposite trailing edge with a round shape or a tapering
tail-like process that is probably similar to a retraction fibre (Fig. 4A, B).
N. Nakatsuji, A. C. Gould and K. E. Johnson
Cell migration in Ambystoma gastrulae
213
Fig. 6 shows the outline and polarity of the cells in Fig. 3, where their direction
could be determined with certainty from scanning electron micrographs. The omitted
remaining cells were obscured by other cells or were without apparent polarity. Strong
orientation is obvious not only among the leading cells in the front line of the migrating
cell group, but also among the trailing cells behind the front line. Out of 367 migrating
mesodermal cells observed in scanning electron micrographs of four embryos, 138
cells were partially overlapped by other cells so that their polarity could not be
determined. Out of the remaining 229 cells, 63 cells had not enough morphological
features to show their polarity. Thus, the direction could be determined with certainty
on 166 cells. In this group of cells, 81 % were directed in a quadrant toward the animal
pole, 14% to the left or right of it, and 5% away from it (Table 1). When the direction
was scored either toward or away from the animal pole, 93 % of the cells were directed
to some extent toward the animal pole (Table 1).
Extracellular fibrils on the inner surface of the ectodermal layer
Scanning electron micrographs at higher magnifications reveal the presence of a
network of fine extracellular fibrils all over the inner surface of the ectodermal layer
(Fig. 4B-F), including the surface around the animal pole far from the migrating
mesodermal cells. A careful examination shows that the fibrils are absent on the surface of the migrating mesodermal cells (Figs. 4B, 5). All the fibrilar structures observed in low-magnification micrographs (Fig. 4A) are filopodia and blebs attached to
the cell surface (Fig. 5). In the area around the animal pole, the fibrils make bundles
and aggregates centred on each ectodermal cell and diverging toward the cell periphery
(Fig. 4 c). The fibrils often continue across cell borders onto the neighbouring ectodermal cell surface (Fig. 4D). This tendency to extend across cell boundaries clearly
shows that these are extracellular structures. The fibrils are uniform in size with
Fig. 4. Scanning electron micrographs of the inner surface of the ectodermal layer and
the migrating mesodermal cells. Bars: A, io/*m;B-F, 1 ftm. A. A migrating mesodermal
cell with typical features that suggest direction of the cell movement: a lamellipodium
at the leading end (arrow) and a tapering tail-like process at the trailing end. The
animal pole is to the top of the picture, B. A higher magnification view of the lamellipodium in A. The surface of the mesodermal cell body and lamellipodium is smooth,
but the inner surface of the ectodermal layer has a network of fine fibrils. Filopodia
and short projections from the lamellipodium are attached to the fibrils (arrows),
c. The inner surface of the ectodermal layer around the animal pole. The fibrils make
bundles and aggregates at near the centre of one cell, and diverge toward the cell
periphery, then continue to the neighbouring cell. D. A tilted view of the fibrils that
cross the border between the ectodermal cells. Some fibrils are clearly continuous
across the border (arrow), though many fibrils were broken at the border during
preparation of the specimens. B. A view of the fibrils and a few filopodia of the mesodermal cells. The tips of the filopodia attach to the fibrils (single arrows). Another
filopodium (double arrow) apparently follows a fibril that is probably continuous
between arrowheads under the filopodium. F. A high-magnification view of two filopodia (arrows), from whose tips many fibrils diverge, suggesting that the fibrils are attached to the tips and pulled by the filopodia. Note the granular substructure of the
fibrils.
214
N. Nakatsuji, A. C. Gould and K. E. Johnson
Fig. 5. A high-magnification view of the central portion of the migrating mesodermal
cell shown in Fig. 4 A. There arefilopodiaand blebs attached to the cell surface, but no
fine fibrils. The larger size and smoother surface of filopodia make them distinguishable from the fine fibrils (arrows) on the inner surface of the ectodermal layer, which is
shown in the lower portion of the picture. Bar, 10 /*m.
Cell migration in Ambystoma gastrulae
215
Fig. 6. Traced outlines and polarities of the migrating mesodermal cells whose
direction can be determined in Fig. 3. The animal pole is to the left of the figure.
The arrows in the cell outlines were drawn from the middle of the rounded or tapering
ends toward the middle of the flattened ends with lamellipodia that are attached to the
inner surface of the ectodermal layer or, in some cells, attached to the other mesodermal cells. Bar, 100 /*m.
apparent thickness of about o-i fim in scanning electron micrographs, and with a
granular substructure (Fig. 4E, F). Frequently, the filopodia attach to and appear to
follow the fibrils (Fig. 4B, E, F). Preliminary studies have shown that the similar fibrils
are present on the inner surface of the ectodermal layer in gastrulae of the urodele
Cynops pyrrhogaster and the anuran Xenopus laevis.
Montages of scanning electron micrographs at a higher magnification (Fig. 7) of
the inner surface of the ectodermal layer, just in front of the migrating cells, give an
impression that the network of the extracellular fibrils is aligned along the blastoporeanimal pole axis. The statistical analysis, as described in Materials and Methods, of
the traced fibrils (Fig. 8) of five montages yields alignment ratios greater than unity
on four montages (Table 2). One montage (no. 5) has a marginal value.
N. Nakatsuji, A. C. Gould and K. E. Johnson
2i6
Table i. Direction of the migrating mesodermal cells
0*
e < 45°
Embryo i
21
(84%)
45° £ e < 135°
3 (12%)
e a 135°
i
(4%)
6 < 9O°
24 (96%)
o g9O°
I (4%)
Embryo 2
Embryo 3
Embryo 4
Total
36 (74 %)
37 (80 %)
40 (87 %)
(a quadrant toward the animal pole)
134 (81 %)
9(18%)
6(13%)
S(n%)
(quadrants to the left or right)
23(14%)
3 (7 %)
1 (2 %)
4 (8 %)
(a quadrant away from the animal pole)
9 (S %)
45 (92 %)
43 (93 %)
43 (93 %)
(to some extent toward the animal pole)
155 (93 %)
4 (8 %)
3 (7 %)
3 (7 %)
11 (7 %)
(to some extent away from the animal pole)
• Angle made by the polarity of the cells and the direction toward the animal pole, measured
as shown in Fig. 1.
t Cell number, and percentage of the total cells whose polarity could be determined.
DISCUSSION
Directionality of the migrating mesodermal cells
One possible way of directing movements of presumptive mesodermal cells toward
the animal pole away from the blastopore is the contact paralysis of locomotory
organelles and contact inhibition of movement that would cause dispersion from a
place of high cell density (blastopore) toward a place of low cell density (animal pole).
The existence of the contact paralysis in vitro has been shown in Rana (Johnson, 1976)
and recently in Xenopus cells (Nakatsuji & Johnson, 1982).
In addition to the contact inhibition, cell movement might be guided by a more
specific cue such as contact guidance or haptotaxis. The striking orientation of the
migrating mesodermal cells in Ambystoma and Xenopus (Nakatsuji & Johnson, 1982)
argues in favour of a specific orienting mechanism. Contact guidance, where cells
move aligned along substratum features (Weiss, 1945), might be provided by
the aligned extracellular fibrils on the substratum, along the blastopore-animal
pole axis. Cells in culture have been shown to adhere preferentially to microheterogeneities in their substratum and even move in a directed fashion along gradients
of substratum adhesiveness. Carter (1965) called this behaviour haptotaxis. Perhaps
the extracellular fibrils promote preferential adhesion of locomotory organelles and
direct cell migration in our system as well. With microsurgery, Cooke (1972) showed
Fig. 7. A montage scanning electron micrograph (no. 1 in Table 2) of the inner surface
of the dorsal ectodermal layer ahead of a migrating mesodermal cell (m) at the bottom
of the picture. Orientation of the network of the extracellular fibrils in such a montage
was analysed. The animal pole is to the top of the picture, and the blastopore to the
bottom. Bar, 10 /tm.
Cell migration in Ambystoma gastrulae
217
m
CEL 56
N. Nakatsuji, A. C. Gould and K. E. Johnson
2l8
Fig. 8. Traces of the extracellular fibrils in Fig. 7. This figure gives the impression
that the fibrils are aligned along the blastopore—animal pole axis, which is supported by the statistical analysis. The alignment ratio of this tracing is 2-0 (Table 2).
Blackened areas show aggregates and bundles of the fibrils. In such areas, only borderlines were used for the determination of the slope. Bar, 10 fun. ap, animal pole.
Table 2. Analysis of the orientation of the extracellular fibrils
Number of intersections
Montage
no.
i
2
A*, \y/x\ 1> ,
B\, \yfx\ < 1
603
493
615
484
292
A/B
2-O
437
3
IS
349
4
1020
922
S
-I
• Intersections where the slope of the fibril is within 45° from I the
blastopore-animal
pole axis.
t Intersections where the slope of the fibril is within 45° from the perpendicular line to the
blastopore-animal pole axis.
Cell migration in Ambystoma gastrulae
A
B
219
C
ap
bp
Fig. 9. Diagrams showing how a uniform network without any alignment (A) could
transform into a network aligned along the blastopore(6£)-animal pole (ap) axis
(B, C). The cause of alignment in B is the traction force from the locomotory mesodermal cells, while the cause in c is a stretching of the ectodermal cell layer to which
the network is attached.
that a rotation of 1800 of the ectodermal cell layer ahead of the migrating mesodermal
cells resulted in the development of apparently normal gastrulae and neurulae. This
result argues against a gradient of the adhesiveness toward the animal pole, but it
does not rule out the possibility of aligned structures along the meridian lines.
Rotation of the ectodermal layer by 900 would test the role of such aligned structures
in controlling cell migration.
The pioneering studies of the contact inhibition of movement (Abercrombie &
Heaysman, 1954; Abercrombie, 1961) contain some data on how strongly cells are
directed when the contact inhibition of movement is working for the control of the
cell movement away from an explant. Abercrombie & Heaysman (1954) described the
direction of the cell movement in the outgrowth of fibroblasts from explants. They
scored all the movements into four quadrants: away from the explant, toward it and
to the left or right of it. Their results show that 47% of the movements are in a
quadrant away from the explant, 49 % to the left or right, and 4% toward the explant.
Abercrombie (1961) measured the frequency of the movements that were at least to
some extent directed away from the explant. This frequency ranged from 62 to 87 %
when the fibroblast had contacts with from o to 6 other cells.
In Ambystoma gastrulae, among the mesodermal cells whose polarity could be determined using morphological criteria, 81 % were in a quadrant away from the blastopore, and 93 % were directed away from it at least to some extent. The presumptive
mesodermal cells from amphibian gastrulae have been shown to move in the direction
of the dominant lamellipodium in vitro (Johnson, 1976; Nakatsuji & Johnson, 1982).
These results suggest a stronger orientation in the migrating mesodermal cells than
in the outgrowth of fibroblasts, unless those mesodermal cells, whose polarity could
not be determined morphologically, were in fact moving predominantly in the direc8-2
220
N. Nakatsuji, A. C. Gould and K. E. Johnson
tions other than toward the animal pole. This appears unlikely. Rather, they probably
represent transient periods in cell locomotion after the cell body has contracted toward
the leading edge but before the re-advancement of a leading lamellipodium. Under
these circumstances, the cells whose polarity could be determined are representative
of cell orientation for migrating mesodermal cells. The mesodermal cells are strongly
oriented not only on the front line of the migration, but also in the middle of the cell
group where a cell has similar spaces in front and behind. This also suggests the presence of orienting factors in addition to the contact inhibition of movement.
Extracellular matrix fibrils as a possible factor controlling cell movement
There is a substantial amount of synthesis of the extracellular matrix materials in
amphibian gastrulae (Johnson, iqyja-d, 1978; Kaska & Triplett, 1980). Biochemical
analysis using Rana pipiens embryos has shown that they are large molecules with
protein portions and long chains of sugars including galactose, mannose, glucose and
fucose (Johnson, 1977^, 1978). Extracellular matrix materials isolated from gastrulae
were shown to promote adhesion of the gastrula cells (Johnson, 1981), thus suggesting
their roles in controlling the cell attachment and movement
Transmission electron micrographs have shown, however, only scattered granular
materials between the ectodermal layer and the migrating mesodermal cells in urodele
gastrulae (Nakatsuji, 1975), but no definite layer such as the basal lamina shown in
chick gastrulae (Trelstad, Hay & Revel, 1967) and sea urchin gastrulae (Gibbins,
Tilney & Porter, 1969). With scanning electron microscopy, Johnson et al. (1979)
showed the presence of the extracellular materials distributed among cells in R. pipiens
gastrulae. The network of the fibrils covering the inner surface of the ectodermal layer,
shown in this report, is the first morphologically distinct extracellular structure
observed in amphibian gastrulae. Probably, these fibrils are equivalent to the similar
ones observed in neurula and tailbud-stage embryos (Karfunkel, 1977), and they may
represent a primitive basement membrane. Such a network could be easily missed in
the transmission electron micrographs, because they would show only scattered crosssections of the fibrils.
The absence of the fibrils on the mesodermal cells suggests that the fibrils are not
artifacts of fixation, but are specific materials present on the inner surface of the
ectodermal layer, and absent on the migrating mesodermal cells. The presence of the
fibrils on the surface near the animal pole in early gastrulae suggests that the fibrils
were produced by the ectodermal cells and not by the mesodermal cells, because the
migrating mesodermal cells are still far from this area at this stage. The close association and attachment of the filopodia of the migrating mesodermal cells to the fibrils
suggest that the fibrils serve as a substratum that promotes adhesion and movement of
the mesodermal cells. The fibrils could initiate cell movement by making the substratum available, and further, guide it by their alignment along the meridional lines,
if the result of the present analysis of a limited area of the ectodermal surface represents the whole embryo.
Even if the ectodermal cells produce the network of fibrils without any alignment,
there are two very probable forces during gastrulation that may cause the alignment
Cell migration in Ambystoma gastrulae
221
along the blastopore-animal pole axis (Fig. 9). First, the traction of migrating mesodermal cells could generate an orientation of fibrils along the meridional lines, in a
manner similar to that used by fibroblasts to generate orientation of collagen fibrils
(Harris, Stopak & Wild, 1981). Second, the expansion of the dorsal marginal-zone
cell layer predominantly along the meridional lines (Keller, 1978, 1980), and accompanying mechanical tension in that direction (Beloussov, Dorfman & Cherdantzev,
1
975)> could cause stretching of the network and alignment of the fibrils. Fig. 9 shows
schematic models of these fibril alignments.
We thank Dr A. K. Harris for his help with collecting salamander eggs. This work was
supported by NIH Grant HD11634 to K.E.J.
REFERENCES
M. (1961). The bases of the locomotory behaviour of fibroblasts. Expl Cell Res.
(suppl.) 8, 188-198.
ABERCROMBIE, M. & HEAYSMAN, J. E. M. (1954). Observations on the social behaviour of cells
in tissue culture. II. ' Monolayering' of fibroblasts. Expl Cell Res. 6, 293-306.
BELOUSSOV, L. V., DORFMAN, J. G. & CHERDANTZEV, V. G. (1975). Mechanical stresses and
morphological patterns in amphibian embryos. J. Embryol. exp. Morph. 34, 559-574.
CARTER, S. B. (1965). Principles of cell motility: the direction of cell movement and cancer
invasion. Nature, Lond. 208, 1183-1187.
COOKE, J. (1972). Properties of the primary organization field in the embryo of Xenopus laevis.
III. Retention of polarity in cell groups excised from the region of the early organizer.
J. Embryol. exp. Morph. a8, 47-56.
COOKE, J. (1975). Local autonomy of gastrulation movements after dorsal lip removal in two
anuran amphibians. J'. Embryol. exp. Morph. 33, 147-157.
GIBBINS, J. R., TILNEY, L. G. & PORTER, K. R. (1969). Microtubules in the formation and
development of the primary mesenchyme in Arbacia punctulata. I. The distribution of
microtubules. J. Cell Biol. 41, 201-226.
HARRIS, A. K., STOPAK, D. & WILD, P. (1981). Fibroblast traction as a mechanism for collagen
morphogenesis. Nature, Lond. 290, 249-251.
HOLTFRETER, J. (1943). A study of the mechanics of gastrulation. Part I. J. exp. Zool. 94,
261-318.
HOLTFRETER, J. (1944). A study of the mechanics of gastrulation. Part II. J. exp. Zool. 95,
ABERCROMBIE,
171-212.
JOHNSON,
K. E. (1976). Ruffling and locomotion in Rana pipiens gastrula cells. Expl Cell Res.
101, 7i-77-
K. E. (1977a). Changes in the cell coat at the onset of gastrulation in Xenopus laevis
embryos..?, exp. Zool. 199, 137-142.
JOHNSON, K. E. (19776). Extracellular matrix synthesis in blastula and gastrula stages of normal
and hybrid frog embryos. I. Toluidine blue and lanthanum staining. J. Cell Sci. 25, 313-322.
JOHNSON, K. E. (1977 c). Extracellular matrix synthesis in blastula and gastrula stages of normal
and hybrid frog embryos. II. Autoradiographic observations on the sites of synthesis and
mode of transport of galactose- and glucosamine-labelled materials. J. Cell Sci. 25, 323-334.
JOHNSON, K. E. (1977^). Extracellular matrix synthesis in blastula and gastrula stages of normal
and hybrid frog embryos. III. Characterization of galactose- and glucosamine-labelled
materials. J. Cell Set. 25, 335~354JOHNSON, K. E. (1978). Extracellular matrix synthesis in blastula and gastrula stages of normal
and hybrid frog embryos. IV. Biochemical and autoradiographic observations on fucose-,
glucose-, and mannose-labelled materials. J'. Cell Set. 33, 100-136.
JOHNSON, K. E. (1981). Normal frog gastrula extracellular materials serve as a substratum for
normal and hybrid cell adhesion when covalently coupled with CNBr-activated Sepharose
beads. Cell Different. 10, 47-55.
JOHNSON,
222
N. Nakatsuji, A. C. Gould and K. E. Johnson
K. E., SILVER, M. H. & KELLEY, R. O. (1979). Scanning electron microscopy of
changes in cell shape and extracellular matrix in normal and interspecific hybrid frog
embryos. In Scanning Electron Microscopy/1979/III (ed. R. P. Becker & O. Johari), pp. 517526. AMC O'Hare: Scanning Electron Microscopy, Inc.
KARFUNKEL, P. (1977). SEM analysis of amphibian mesoderm migration. Roux Arch, devl
Biol. 181, 31-40.
KASKA, D. D. & TRIPIETT, E. L. (1980). Glycoprotein secretion by isolated Rana pipiens
gastrula chordamesoderm. Cell Different. 9, 281-290.
KELLER, R. E. (1978). Time-lapse cinemicrographic analysis of superficial cell behavior
during and prior to gastrulation in Xenopus laevis. J. Morph. 157, 223-248.
KELLER, R. E. (1980). The cellular basis of epiboly: An SEM study of deep-cell rearrangement
during gastrulation in Xenopus laevis. J. Embryol. exp. Morph. 60, 2oi-234.
KELLER, R. E. (1981). An experimental analysis of the role of bottle cells and the deep marginal
zone in gastrulation of Xenopus laevis. J. exp. Zool. 316, 81-101.
KELLER, R. & SCHOENWOLF, G. C. (1977). An SEM study of cellular morphology, contact and
arrangement as related to gastrulation in Xenopus laevis. Roux Arch, devl Biol. i8a, 165-186.
KUBOTA, H. Y. & DURSTON, A. J. (1978). Cinematographical study of cell migration in the
opened gastrula of Ambystoma mexicamtm. J. Embryol. Exp. Morph. 44, 71-80.
LOFBERG, J., AHLFORS, K. & FALLSTRQM, C. (1980). Neural crest cell migration in relation to
extracellular matrix organization in the embryonic axolotl trunk. Devl Biol. 75, 148—167.
NAKATSUJI, N. (1974). Studies on the gastrulation of amphibian embryos: pseudopodia in the
gastrula of Bufo bufo japonicus and their significance to gastrulation. J. Embryol. exp. Morph.
3a, 79S-8O4NAKATSUJI, N. (1975). Studies on the gastrulation of amphibian embryos: Light and electron
microscopic observation of a urodele Cynops pyrrhogaster. J. Embryol. exp. Morph. 34,
669-685.
NAKATSUJI, N. (1976). Studies on the gastrulation of amphibian embryos: Ultrastructure of
the migrating cells of anurans. Roux Arch, devl Biol. 180, 229-240.
NAKATSUJI, N. (1979). Effects of injected inhibitors of microfilament and microtubule function
on the gastrulation movement in Xenopus laevis. Devl Biol. 68, 140-150.
NAKATSUJI, N. & JOHNSON, K. E. (1982). Cell locomotion in vitro by Xenopus laevis gastrula
me8odermal cells. Cell Motil. 2 (In Press).
RHUMBLER, L. (1902). Zur Mechanik des Gastrulationsvorganges, insbesondere der Invagination. Eine entwicklungsmechanische Studie. Wilhelm Roux. Arch. EntwMech. Org. 14,
401-476.
RUFFINI, A. (1925). Fisiogenia. Milano: Francesco Vallardi.
RUGH, R. (1962). Experimental Embryology, 3rd edn, Minneapolis: Burgess.
TRELSTAD, R. L., HAY, E. D. & REVEL, J.-P. (1967). Cell contact during early morphogenesis
in the chick embryo. Devl Biol. 16, 78-106.
WEISS, P. (1945). Experiments on cell and axon orientation in vitro: the role of colloidal exudates in tissue organization. J. exp. Zool. 100, 353-386.
JOHNSON,
{Received 7 December 1981)