* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Plant Respiration
Radical (chemistry) wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Biochemical cascade wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Metalloprotein wikipedia , lookup
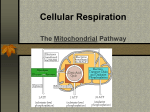
Mitochondrion wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Photosynthesis wikipedia , lookup
Phosphorylation wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Light-dependent reactions wikipedia , lookup
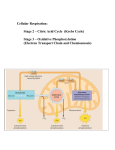
Electron transport chain wikipedia , lookup
Microbial metabolism wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Biochemistry wikipedia , lookup
Plant Respiration Question 1. Differentiate between (a) Respiration and Combustion (b) Glycolysis and Krebs’ cycle (c) Aerobic respiration and Fermentation Answer:(a) Respiration takes place in cells of living beings, while combustion can take place anywhere. Respiration is highly controlled process while combustion cannot be controlled beyond certain level. Both of them require oxygen and are exothermic reaction. Both the processes change chemical energy to heat energy. (b) Glycolysis is a metabolic pathway that is found in the cytoplasm of cells in all living organisms and is anaerobic, or doesn't require oxygen. The process converts one molecule of glucose into two molecules of pyruvate, and makes energy in the form of two net molecules of ATP. Citric acid Cycle or Krebs Cycle:- When oxygen is present, acetyl-CoA is produced from the pyruvate molecules created from glycolysis. Once acetyl-CoA is formed, two processes can occur, aerobic or anaerobic respiration. When oxygen is present, the mitochondria will undergo aerobic respiration which leads to the Krebs cycle. However, if oxygen is not present, fermentation of the pyruvate molecule will occur. In the presence of oxygen, when acetyl-CoA is produced, the molecule then enters the citric acid cycle (Krebs cycle) inside the mitochondrial matrix, and gets oxidized to CO2 while at the same time reducing NAD to NADH. NADH can be used by the electron transport chain to create further ATP as part of oxidative phosphorylation. To fully oxidize the equivalent of one glucose molecule, two acetyl-CoA must be metabolized by the Krebs cycle. Two waste products, H 2O and CO2, are created during this cycle. The citric acid cycle is an 8-step process involving 8 different enzymes. Throughout the entire cycle, acetyl-CoA changes into citrate, isocitrate, α-ketoglutarate, succinylCoA, succinate, fumarate, malate, and finally, oxaloacetate. The net energy gain from one cycle is 3 NADH, 1 FADH, and 1 ATP. Thus, the total amount of energy yield from one whole glucose molecule (2 pyruvate molecules) is 6 NADH, 2 FADH, and 2 ATP. (c) Aerobic respiration Aerobic respiration is the main means by which both plants and animals utilize energy in the form of organic compounds that was previously created through photosynthesis. Respiration requires oxygen in order to generate energy (ATP). It is the preferred method of pyruvate breakdown from glycolysis and requires that pyruvate enter the mitochondrion in order to be fully oxidized by the Krebs cycle. The product of this process is energy in the form of ATP (Adenosine Triphosphate), by substrate-level phosphorylation, NADH and FADH 2. Anaerobic Respiration or Fermentation Without oxygen, pyruvate is not metabolized by cellular respiration but undergoes a process of fermentation. The pyruvate is not transported into the mitochondrion, but remains in the cytoplasm, where it is converted to waste products that may be removed from the cell. This serves the purpose of oxidizing the hydrogen carriers so that they can perform glycolysis again and removing the excess pyruvate. This waste product varies depending on the organism. In skeletal muscles, the waste product is lactic acid. This type of fermentation is called lactic acid fermentation. In yeast, the waste products are ethanol and carbon dioxide. This type of fermentation is known as alcoholic or ethanol fermentation. The ATP generated in this process is made by substrate phosphorylation, which is phosphorylation that does not involve oxygen. Question 2. What are respiratory substrates? Name the most common respiratory substrate. Answer:- Compounds which are oxidized during respiration are called respiratory substrates. It is obvious that carbohydrates are the most common respiratory substrates. Question 3. Give the schematic representation of glyolysis? Answer:- Question 4. What are the main steps in aerobic respiration? Where does it take place? Answer: • Prior to entering the Krebs Cycle, pyruvate must be converted into acetyl CoA (pronounced: acetyl coenzyme A). This is achieved by removing a CO 2 molecule from pyruvate and then removing an electron to reduce an NAD + into NADH. An enzyme called coenzyme A is combined with the remaining acetyl to make acetyl CoA which is then fed into the Krebs Cycle. The steps in the Krebs Cycle are summarized below: • Citrate is formed when the acetyl group from acetyl CoA combines with oxaloacetate from the previous Krebs cycle.. • Citrate is converted into its isomer isocitrate.. • Isocitrate is oxidized to form the 5-carbon α-ketoglutarate. This step releases one molecule of CO2 and reduces NAD+ to NADH2+ . • The α-ketoglutarate is oxidized to succinyl CoA, yielding CO 2 and NADH2+. • Succinyl CoA releases coenzyme A and phosphorylates ADP into ATP. • Succinate is oxidized to fumarate, converting FAD to FADH 2. • Fumarate is hydrolized to form malate. • Malate is oxidized to oxaloacetate, reducing NAD + to NADH2+. Question 5. Give the schematic representation of an overall view of Krebs’ cycle. Answer: Question 6. Explain ETS. Answer: The following steps in the respiratory process are to release and utilize the energy stored in NADH+H+ and FADH 2. This is accomplished when they are oxidised through the electron transport system and the electrons are passed on to O2 resulting in the formation of H 2O. The metabolic pathway through which the electron passes from one carrier to another, is called the electron transport system (ETS) and it is present in the inner mitochondrial membrane. Electrons from NADH produced in the mitochondrial matrix during citric acid cycle are oxidised by an NADH dehydrogenase (complex I), and electrons are then transferred to ubiquinone located within the inner membrane. Ubiquinone also receives reducing equivalents via FADH2 (complex II) that is generated during oxidation of succinate in the citric acid cycle. The reduced ubiquinone (ubiquinol) is then oxidised with the transfer of electrons to cytochrome c via cytochrome bc1 complex (complex III). Cytochrome c is a small protein attached to the outer surface of the inner membrane and acts as a mobile carrier for transfer of electrons between complex III and IV. Complex IV refers to cytochrome c oxidase complex containing cytochromes a and a3, and two copper centres. When the electrons pass from one carrier to another via complex I to IV in the electron transport chain, they are coupled to ATP synthase (complex V) for the production of ATP from ADP and inorganic phosphate. The number of ATP molecules synthesized depends on the nature of the electron donor. Oxidation of one molecule of NADH gives rise to 3 molecules of ATP, while that of one molecule of FADH2produces 2 molecules of ATP. Although the aerobic process of respiration takes place only in the presence of oxygen, the role of oxygen is limited to the terminal stage of the process. Yet, the presence of oxygen is vital, since it drives the whole process by removing hydrogen from the system. Oxygen acts as the final hydrogen acceptor. Unlike photophosphorylation where it is the light energy that is utilised for the production of proton gradient required for phosphorylation, in respiration it is the energy of oxidation-reduction utilised for the same process. It is for this reason that the process is called oxidative phosphorylation. The energy released during the electron transport system is utilised in synthesising ATP with the help of ATP synthase (complex V). This complex consists of two major components, F1 and F0. The F1 headpiece is a peripheral membrane protein complex and contains the site for synthesis of ATP from ADP and inorganic phosphate. F 0 is an integral membrane protein complex that forms the channel through which protons cross the inner membrane. The passage of protons through the channel is coupled to the catalytic site of the F1 component for the production of ATP. For each ATP produced, 2H+ passes through F0 from the intermembrane space to the matrix down the electrochemical proton gradient. Question 7. What are the assumptions made during the calculation of net gain of ATP? Answer: It is possible to make calculations of the net gain of ATP for every glucose molecule oxidised; but in reality this can remain only a theoretical exercise. These calculations can be made only on certain assumptions that: • There is a sequential, orderly pathway functioning, with one substrate forming the next and with glycolysis, TCA cycle and ETS pathway following one after another. • The NADH synthesised in glycolysis is transferred into the mitochondria and undergoes oxidative phosphorylation. • None of the intermediates in the pathway are utilised to synthesise any other compound. • Only glucose is being respired – no other alternative substrates are entering in the pathway at any of the intermediary stages. But this kind of assumptions are not really valid in a living system; all pathways work simultaneously and do not take place one after another; substrates enter the pathways and are withdrawn from it as and when necessary; ATP is utilised as and when needed; enzymatic rates are controlled by multiple means. Yet, it is useful to do this exercise to appreciate the beauty and efficiency of the living system in extraction and storing energy. Hence, there can be a net gain of 36 ATP molecules during aerobic respiration of one molecule of glucose. Question 8. Discuss “The respiratory pathway is an amphibolic pathway.” Answer: Glucose is the favoured substrate for respiration. All carbohydrates are usually first converted into glucose before they are used for respiration. Other substrates can also be respired but then they do not enter the respiratory pathway at the first step. Since respiration involves breakdown as well as synthesis of substrates, the respiratory process involves both catabolism and anabolism. That is why respiratory pathway is considered to be an amphibolic pathway rather than as a catabolic one. Question 9. Define RQ. What is its value for fats? Answer: The ratio of the volume of CO 2 evolved to the volume of O 2 consumed in respiration is called the respiratory quotient (RQ) or respiratory ratio. Question 10. What is oxidative phosphorylation? Answer: Oxidative phosphorylation is a metabolic pathway that uses energy released by the oxidation of nutrients to produce adenosine triphosphate (ATP). Although the many forms of life on earth use a range of different nutrients, almost all carry out oxidative phosphorylation to produce ATP, the molecule that supplies energy to metabolism. This pathway is probably so pervasive because it is a highly efficient way of releasing energy, compared to alternative fermentation processes such as anaerobic glycolysis. During oxidative phosphorylation, electrons are transferred from electron donors to electron acceptors such as oxygen, in redox reactions. These redox reactions release energy, which is used to form ATP. In eukaryotes, these redox reactions are carried out by a series of protein complexes within mitochondria, whereas, in prokaryotes, these proteins are located in the cells' inner membranes. Thes e linked sets of enzymes are called electron transport chains. In eukaryotes, five main protein complexes are involved, whereas in prokaryotes many different enzymes are present, using a variety of electron donors and acceptors.