* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Clinical and Neuropathological Features of
Neuroplasticity wikipedia , lookup
Metastability in the brain wikipedia , lookup
Cognitive neuroscience wikipedia , lookup
Biochemistry of Alzheimer's disease wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Synaptic gating wikipedia , lookup
Neuroeconomics wikipedia , lookup
Aging brain wikipedia , lookup
National Institute of Neurological Disorders and Stroke wikipedia , lookup
Optogenetics wikipedia , lookup
Neuroanatomy wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
Neurogenomics wikipedia , lookup
Channelrhodopsin wikipedia , lookup
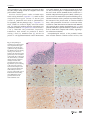
Cerebellum DOI 10.1007/s12311-014-0643-7 LETTER TO THE EDITOR Clinical and Neuropathological Features of Spastic Ataxia in a Spanish Family with Novel Compound Heterozygous Mutations in STUB1 Conceição Bettencourt & Justo García de Yébenes & José Luis López-Sendón & Orr Shomroni & Xingqian Zhang & Shu-Bing Qian & Ingrid M. C. Bakker & Sasja Heetveld & Raquel Ros & Beatriz Quintáns & María-Jesús Sobrido & Marianna R. Bevova & Shushant Jain & Marianna Bugiani & Peter Heutink & Patrizia Rizzu # Springer Science+Business Media New York 2015 Hereditary ataxias are clinically and genetically heterogeneous neurodegenerative disorders. Although many ataxia genes have been identified, about 50 % of cases await the identification of the genetic cause [1]. High-throughput sequencing, namely whole-exome sequencing (WES), has changed the paradigm of genetic analysis of rare heterogeneous Mendelian disorders [2], and allowed the identification of STUB1 mutations as the cause of autosomal recessive ataxia syndromes [3–7]. We report on clinical and pathological features of a family with spastic ataxia due to novel STUB1 Electronic supplementary material The online version of this article (doi:10.1007/s12311-014-0643-7) contains supplementary material, which is available to authorized users. C. Bettencourt Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK C. Bettencourt Institute for Molecular and Cell Biology (IBMC), University of Porto, Porto, Portugal C. Bettencourt (*) Center of Research in Natural Resources (CIRN) and Department of Biology, University of the Azores, Ponta Delgada, Portugal e-mail: [email protected] J. G. de Yébenes : J. L. López-Sendón : R. Ros Department of Neurology, Hospital Ramón y Cajal, Madrid, Spain B. Quintáns : M.<J. Sobrido Fundación Pública Galega de Medicina Xenómica and Health Research Institute of Santiago de Compostela (IDIS), Clinical Hospital of Santiago de Compostela-SERGAS, Santiago de Compostela, Spain M. R. Bevova European Research Institute for the Biology of Ageing, Groningen, The Netherlands S. Jain : P. Heutink : P. Rizzu German Center for Neurodegenerative Diseases, Tübingen, Germany O. Shomroni : I. M. C. Bakker : S. Heetveld : M. R. Bevova : S. Jain : P. Heutink : P. Rizzu Department of Clinical Genetics, Neuroscience Campus Amsterdam, VU University Medical Center, Amsterdam, The Netherlands M. Bugiani Department of Pediatrics, Child Neurology, Neuroscience Campus Amsterdam, VU University Medical Center, Amsterdam, The Netherlands X. Zhang : S.<B. Qian Division of Nutritional Sciences, Cornell University, Ithaca, NY, USA M. Bugiani Department of Pathology, VU University Medical Center, Amsterdam, The Netherlands Cerebellum mutations identified by WES. The study had ethics committee approval, and all subjects provided written informed consent. Two out of three siblings born to nonconsanguineous healthy Spanish parents were affected (Fig. 1), suggesting autosomal recessive inheritance. The older sibling (proband) was a woman who presented progressive incoordination of movement and unsteadiness since her 20s. Initial examination revealed upper and lower limb dysmetria, dysdiadochokinesia, cerebellar dysarthria, and saccadic ocular pursuit with increased tendon reflexes in the four limbs. Assessment of other neurological systems was initially unremarkable. Ataxia and pyramidal tract dysfunction became more prominent, and unassisted walking became impossible in her late 30s. She later presented with cognitive impairment that progressed to dementia, mild generalized choreatric movements, and spontaneous myoclonus. At age 52, she was bedridden and suffered severe neurogenic dysphagia, which led to aspiration pneumonia and death. Her brother was diagnosed with ataxia with retained reflexes at age 22. Subsequent neurological examinations revealed a more generalized cerebellar deficit causing dysarthria, limb ataxia, wide-base gait, and hyperactive tendon reflexes with lower limb spasticity. In his 40s, he exhibited progressive neurological deterioration with generalized ataxia, spasticity, myoclonus, and cognitive deterioration that led to dementia. He died at age 46 due to neurological complications. Metabolic and endocrine studies as well as electromyogram and nerve conduction velocities were normal in both patients. MRI brain scans revealed progressive midline and hemispheric cerebellar atrophy. We did not find any signs Fig. 1 a Pedigree of a Spanish ataxia family showing the STUB1 mutations identified in each family member. The arrow points to the proband. WT wild-type; M1: heterozygous for the c.633G>A, p.Met211Ile mutation; M2: heterozygous for the c.712G>T, p.Glu238Ter mutation *Whole-exome sequencing was performed for these individuals. b STUB1 mutations found in the ataxia family, highlighting the conservation between species at those positions. c CHIP protein domain structures (tetratricopeptide repeat domains—TPR and U-Box domain) with all STUB1 mutations described in the literature depicted (in red are highlighted the mutations found in our family) Cerebellum of hypogonadism in any of the patients. The parents (in their 80s), the younger sibling, and the two daughters of the older patient remain unaffected. Previous classical genetic studies excluded major ataxia-causing mutations (Appendix e–1). WES revealed compound heterozygous variants in STUB1 gene (c.633G>A, p.Met211Ile and c.712G>T, p.Glu238Ter) to be segregating with the phenotype (Fig. 1, Appendix e–1). These variants are located in highly conserved residues (Fig. 1b), with a 100 vertebrates basewise conservation score by PhyloP (available at www.genome.ucsc.edu) of 8.8 and 9. 7 for p. Met211Ile and p.Glu238Ter, respectively. Furthermore, both variants are predicted as disease causing (>0.99) by Mutation Taster [8], and are not present in ∼560 population-matched control chromosomes Fig. 2 Neuropathological examination of an ataxia patient with compound heterozygous mutations in the STUB1 gene (p.Met211Ile and p.Glu238Ter). a, b Hematoxylin-eosin staining showing severe decrease of Purkinje cells and neurons of the granular layer in the cerebellum of the patient (b) as compared to the control brain (a). c, d CHIPlabeling showing cytoplasmic staining in neurons of the paraventricular nuclei of the hypothalamus (c) and extending to the apical part of axons in neurons of the temporal cortex (d). e Varicosities labeled with CHIP antibody in the nerve processes of the frontal cortex. f Predominant nuclear staining with CHIP antibody in the pyramidal neurons of the medio-entorhinal cortex or in public databases. By screening a Spanish ataxia cohort (N=185), no homozygous or compound heterozygous variants were found. STUB1 (OMIM# 607207) encodes the Cterminal of Hsp70-interacting protein (CHIP), a dimeric protein involved in the metabolism of protein misfolding. The p. Glu238Ter mutation causes a premature stop codon leading to the truncation of the protein and/or to nonsense-mediated decay, while p.Met211Ile is located in a conserved residue, essential for CHIP dimerization and function. Both HEK293 cells expressing these mutants (Supplementary Fig. e–1) and proband’s fibroblasts (reported elsewhere [9]) show decreased levels of CHIP when compared with controls, supporting a loss-of-function mechanism. Neuropathological analysis of the proband’s brother brain revealed dramatic cerebellar cortical cell loss, with Cerebellum severe loss of Purkinje cells and neurons of the granular layer, accompanied by reactive Bergmann gliosis (Fig. 2a, b). Staining with CHIP antibody showed mainly cytoplasmic diffuse reactivity in neurons, often extending to dendrites and axons (Fig. 2c, d), and also in astrocytes. Some swollen axonal processes in the frontal white matter stained as well (Fig. 2e). Remarkably, intense nuclear staining in neurons of the deep layers of the frontal cortex and in the pyramidal stellate neurons of medio-entorhinal cortex, with only mild cytoplasmic staining, was observed in this patient (Fig. 2f). Staining with HSP70-antibody showed an overlap with CHIP in all investigated brain tissues. P62-antibody stained a few neuronal intranuclear inclusions in small neurons of frontal cortex and caudate, and seldom apparently free-lying bodies in the cerebellum and putamen (data not shown). Mutations in STUB1 have been recently described in Gordon Holmes syndrome and in additional autosomal recessive forms of ataxia [3–7]. The phenotype of our patients is similar to most of the previous reports, including an ataxic syndrome with onset in the mid20s, progressive pyramidal tract dysfunction, and myoclonus. We also observed an increasing impairment of memory, language, and executive function leading to dementia in the later stages of the disease, likely related to a delayed involvement of frontal and temporal cortex as revealed by the neuropathological findings. In addition, our patients presented acute and partially reversible episodes of sudden neurological deterioration, unreported in other cases with mutations in the STUB1 gene. All previous reports suggest a classical loss-of-function mechanism of the protein—CHIP, a chaperone widely expressed in the nervous system. In our family, the presence of compound heterozygous mutations and a pedigree compatible with an autosomal recessive inheritance is in line with those reports. Our study adds pathological information and expands the mutational spectrum of STUB1-associated ataxias. This study further reinforces the utility of clinical genomics in the diagnosis of heterogeneous neurodegenerative disorders. Acknowledgments The authors thank all the patients who participate in this study and their families, as well as people involved in the collection of samples; the Netherlands Brain Bank (Amsterdam, the Netherlands) for providing brain tissue of the controls; and A. Ingrassia and Dr W.D. van de Berg for technical support in the immunohistochemistry experiments. This study was supported by the “CIBERNED,” the “Ministerio de Economía y Competitividad—“Gobierno de España” (AIB2010PT00182) and “Instituto de Salud Carlos III (PI12/00742),” and the “Xunta de Galicia, Dirección Xeral de Investigación, Desenvolvemento e Innovación” (10 PXIB 9101 280 PR). CB was recipient of a postdoctoral fellowship from the “Fundação para a Ciência e a Tecnologia”—FCT [SFRH/BPD/63121/2009] until January 2013, and is presently supported by the Medical Research Council (UK). Conflict of Interest The authors declare no conflict of interest. References 1. Hersheson J, Haworth A, Houlden H. The inherited ataxias: genetic heterogeneity, mutation databases, and future directions in research and clinical diagnostics. Hum Mutat. 2012;33(9):1324–32. 2. Bettencourt C, Lopez-Sendon J, Garcia-Caldentey J, Rizzu P, Bakker I, Shomroni O, et al. Exome sequencing is a useful diagnostic tool for complicated forms of hereditary spastic paraplegia. Clinical genetics. 2014;85(2):154–8. 3. Shi CH. Schisler JC. Tan S, Song B, McDonough H, et al. Ataxia and hypogonadism caused by the loss of ubiquitin ligase activity of the U box protein CHIP. Human molecular genetics. 2014;23(4):1013–24. 4. Shi Y, Wang J, Li JD, Ren H, Guan W, He M, et al. Identification of CHIP as a novel causative gene for autosomal recessive cerebellar ataxia. PLoS One. 2013;8(12):e81884. 5. Depondt C, Donatello S, Simonis N, Rai M, van Heurck R, Abramowicz M, et al. Autosomal recessive cerebellar ataxia of adult onset due to STUB1 mutations. Neurology. 2014;82(19):1749–50. 6. Cordoba M, Rodriguez-Quiroga S, Gatto EM, Alurralde A, Kauffman MA. Ataxia plus myoclonus in a 23-year-old patient due to STUB1 mutations. Neurology. 2014;83(3):287–8. 7. Synofzik M, Schule R, Schulze M, Gburek-Augustat J, Schweizer R, Schirmacher A, et al. Phenotype and frequency of STUB1 mutations: next-generation screenings in Caucasian ataxia and spastic paraplegia cohorts. Orphanet j rare dis. 2014;9(1):57. 8. Schwarz JM, Cooper DN, Schuelke M, Seelow D. MutationTaster2: mutation prediction for the deep-sequencing age. Nat Methods. 2014;11(4):361–2. 9. Casarejos MJ, Perucho J, Lopez-Sendon JL. Garcia de Yebenes J, Bettencourt C, Gomez A, et al. Trehalose Improves Human Fibroblast Deficits in a New CHIP-Mutation Related Ataxia. PLoS One. 2014;9(9):e106931.