* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Introduction to Biotechnology
Nicotinamide adenine dinucleotide wikipedia , lookup
Metalloprotein wikipedia , lookup
Phosphorylation wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Photosynthesis wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Biochemistry wikipedia , lookup
Microbial metabolism wikipedia , lookup
Electron transport chain wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
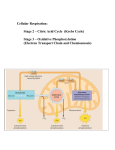
Chapter 10 Catabolism: Energy Release and Conservation Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or 1 Microbial Metabolism A. Basic Concepts of Metabolism B. Glycolytic Pathways C. Fermentation D. Respiration E. Photosynthesis F. Chemolithotrophy G. The Nitrogen Cycle Recap … Metabolism Anabolism and catabolism are intimately linked by energy coupling Energy coupling means that the energy “generated” by catabolic processes is harnessed by cells to perform anabolic processes “The metabolic pathways intersect in such a way that energy released from the ‘downhill’ reactions of catabolism can be used to drive the 'uphill' reactions of the anabolic pathways. This transfer of energy from catabolism to anabolism is called energy coupling.” Exergonic reaction Exergonic reaction An exergonic reaction net-generates (gives off) energy (e.g., heat) That is, the products of such a reaction possess less stored energy than do the reactants Endergonic reaction Endergonic reaction An endergonic reaction is one that requires a net input of energy in order to proceed The products of endergonic reactions possess more energy than do the reactant ..REDOX Reduction and Oxidation Reduction and oxidation always occur together. In a reduction-oxidation reaction (redox reaction), one substance gets reduced, and another substance gets oxidized. The thing that gets oxidized is called the electron donor, and the thing that gets reduced is called the electron acceptor. …Catabolism Chemoorganotrophs Animals and many microorganisms are Chemoorganotrophs i.e use of organic molecules as the source of energy. Molecules that supplies them with energy also supplies them with carbon and electrons ..catabolism Chemoheteroptrophs also referred as Chemoorganotrophs can use one or more of the following catabolism: Aerobic respiration Anaerobic Respiration or Fermentation Chemoorganic Fueling Processes −E.g Respiration Most respiration involves use of an electron transport chain which uses exogenous electron acceptor Aerobic respiration Anaerobic respiration final electron acceptor is oxygen final electron acceptor such as NO3-, SO42-, CO2, etc. In respiration, as electrons pass through the electron transport chain to the final electron acceptor, a proton motive force (PMF)/potential energy is generated and used to synthesize ATP from ADP and P Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 9 Chemoorganic Fueling Processes −E.g Fermentation Whereas, fermentation, uses an endogenous (from within the cells) electron acceptor usually an intermediate of the pathway used to oxidize the organic energy source (e.g., pyruvate) Does not involve the use of an electron transport chain nor the generation of a proton motive force/potential energy ATP synthesized only by substrate-level phosphorylation Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 10 Glycolytic pathways Four major glycolytic pathways found in different bacteria: Embden-Meyerhoff-Parnas pathway Hexose monophosphate pathway/Pentose Phosphate Also found in most organisms Responsible for synthesis of pentose sugars used in nucleotide synthesis Entner-Doudoroff pathway “Classic” glycolysis Found in almost all organisms Found in Pseudomonas and related genera Phosphoketolase pathway Found in Bifidobacterium and Leuconostoc Fermentation Pyruvic acid is reduced to form organic acids or alcohols. Examples of fermentation pathways Lactic acid fermentation Mixed acid fermentation Found in many bacteria; e.g. Streptococcus cremoris, Lactobacillus acidophilus e.g. Escherichia coli Basis of the methyl red test 2,3-Butanediol fermentation e.g. Enterobacter aerogenes Basis of the Voges-Proskauer reaction …Fermentation Respiration Cellular respiration is a series of chemical and physical processes which together serve to remove potential energy containing electrons from organic compounds, use the energy thus liberated to generate ATP and then donate these now energy-spent electrons to oxygen …Respiration Glucose is oxidized and releases its electrons during cell respiration (electrons associated with H atoms).Sugar gets oxidized (electrons taken off) and oxygen gets reduced (takes electrons) Overall reaction (oxidation of glucose): C6H12O6 + 6O2 (oxygen required) ---> 6CO2 +6H2O + energy + heat Delta G = -686kcal/mol …Respiration Majority of energy (90%) is generated by electron transport chain on inner membrane of mitochondria (oxidative phosphorylation). Small amount of energy created by phosphorylation of ADP during Glycolysis and Krebs cycle The steps of Cellular respiration Respiration includes Glycolysis, Krebs Cycle (TCA or Citric Acid Cycle), and Electron transport chain The steps of Cellular respiration A. Glycolysis (glyco =sugar, lysis = break) Initial breakdown of glucose B. The Krebs cycle /(TCA or Citric Acid Cycle), Further breakdown of glucose yielding electrons and CO2 C. The electron transport chain. where electrons taken from glucose during glycolysis and Krebs cycle are taken;where most of the ATP is finally re-made. The Tricarboxylic Acid Cycle also called citric acid cycle and Kreb’s cycle common in aerobic bacteria, free-living protozoa, most algae, and fungi Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 25 2.Located in the inner membrane of the mitochondrion. 3. Electrons are passed down the electron chain, along the energy gradient, to oxygen Figure 10.8 Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 27 Glycolysis A. Glycolysis (splitting of sugar) occurs in cytoplasm-end product is pyruvate. Pathway has ten steps: first five steps are energy investment, final five steps yield energy. As glucose is oxidized and broken down NAD+ is reduced to NADH (electron carrier). Glycolysis occurs whether or not O2 is present. Glycolysis which occurs in the absence of O2 is termed fermentation. In the presence of O2, NADH is passed on to the electron transport chain and pyruvate is passed into the Krebs Cycle. ...Glycolisis Overview 1. glucose is split into 2 (3C)(Pyruvic acid molecules) sugars 2. the 3C sugars are oxidized and rearranged to produce pyruvate. 3. the oxidation is coupled with NAD+ being reduced to NADH. 4. consists of 2 phases a.energy investment phase (using 2 ATPs per glucose molecule) b. energy yielding phase (making 4 ATPs per glucose molecule via substrate-level phosphorylation.) …Glycolysis Overview 5. other interesting facts about glycolysis: a. occurs in cytosol (ground substance of cell) b. anaerobic (does not need O2,not really a part of respiration). c. Pyruvate enters the mitochondrion through a transport protein. …. GLYCOLYSIS Overview Glucose molecule in the cytosol 2 pyruvic acid molecules (3 carbons), 4 ATP formed (2 ATP used up), 2 NADH/glucose formed. Pyruvic acid & NADH enter mitochondria The First Stage of Glycolysis Glucose (6C is broken down into 2 PGAL's (Phosphoglyceraldehyde - 3Carbon molecules) This requires two ATP's The Second Stage of Glycolysis 2 PGAL's (3C) are converted to 2 pyruvates This creates 4 ATP's and 2 NADH's The net ATP production of Glycolysis is 2 ATP's Formation of acetyl CoA B. Formation of acetyl CoA 1. Intermediate stage between glycolysis and Krebs Cycle. 2. The oxidation of 3C pyruvate to 2C acetyl CoA (with the loss of CO2) coupled with the reduction of NAD+ to NADH. ..Formation of acetyl CoA The Oxidation of Pyruvate to form Acetyl CoA for Entry Into the Krebs Cycle •2 NADH's are generated (1 per pyruvate) •2 CO2 are released (1 per pyruvate) Kreb Cycle Preliminary step is conversion of pyruvate to acetyl CoA. catalyzed by pyruvate dehydrogenase complex (complex of three enzymes) and byproducts of conversion to acetyl CoA are CO2 and NADH. There are eight steps in pathway in the Kreb Cycle Initial step is condensation of acetyl CoA with oxaloacetate and final step which completes the cycle is the decomposition of citrate back to oxaloacetate. Steps in Kreb Cycle Step 1: The unstable bond of acetyl CoA breaks, and the two-carbon acetyl group bonds to the four-carbon oxaloacetate to form six-carbon citrate. Step 2: Citrate is isomerized to isocitrate. Step 3: Two major events occur during this step: -Isocitrate loses CO2 leaving a five-carbon molecule. -The five-carbon compound is oxidized and NAD+ is reduced. ..Steps in Kreb Cycle Step 4: A multi enzyme complex catalyzes: - Removal of CO2 - Oxidation of the remaining four-carbon compound and reduction of NAD+ - Attachment of CoA with a high energy bond to form succinyl CoA. ..Steps in Kreb Cycle Step 5: Substrate-level phosphorylation occurs in a series of enzyme catalyzed reactions: -The high energy bond of succinyl CoA breaks, and some energy is conserved as CoA is displaced by a phosphate group. -The phosphate group is transferred to GDP to form GTP (Guanosine triphospahte) and succinate. GTP donates a phosphate group to ADP to form ATP. Step 6: Succinate is oxidized to fumarate and FAD is reduced. - Two hydrogens are transferred to FAD to form FADH2. - The dehydrogenase that catalyzes this reaction is bound to the inner mitochondrial membrane. ..Steps in Kreb Cycle Step 7: Water is added to fumarate which rearranges its chemical bonds to form malate ..Steps in Kreb Cycle Step 8: Malate is oxidized and NAD+ is reduced. -A molecule of NADH is produced. -Oxaloacetate is regenerated to begin the Kreb cycle again. -Two turns of the Krebs cycle produce two ATPs by substrate-level phosphorylation. -ATP output of respiration results from oxidative phosphorylation. - Reduced coenzymes produced by the Krebs cycle (six NADH and two FADH2 per glucose) carry high energy electrons to the electron transport chain. - The ETC couples electron flow down the chain to ATP synthesis. Figure 10.8-Tri carboxylic Acid Cycle 44 Net Energy Gained Summary of the net energy yield : From glycolysis : 2ATP + 2 NADH From matrix reaction : 2 NADH From Kreb cycle : 2 ATP + 6 NADH + 2 FADH2 Total : 4 ATP + 10 NADH + 2 FADH2 Most of the energy that has been released has been captured in the form of electron transport molecules. malate. NAD binds to malate and leaves as NADH which creates a new oxaloacetate molec . Electron transport chain C. Electron transport chain: • Located on inner membrane of mitochondria. •Composed of three protein complexes in the membrane and two mobile electron carriers (Ubiquinone, Cytochromes). • Electrons passed down chain in series of reductions and oxidation reactions. •Each successive complex has a higher affinity for the electrons. •Oxygen is the ultimate electron acceptor . As electrons are passed along chain, hydrogen (H+) atoms are pumped from the matrix to the inner membrane space of mitochondria. • Exergonic energy created by electron flow powers H+ pumps. The Electron Transport Chain Utilizes NADH and FADH2 formed in the previous steps (kreb’s cycle) Series of electron carriers that operate together to transfer electrons from NADH and FADH2 to a terminal electron acceptor Movement of H+ ions down concentration gradient provides energy to make ATP from ADP and P catalyzed by ATP synthase, an integral membrane protein. It is not known exactly how the ATP synthase using the downhill H+ current to attach inorganic phosphate to ADP. Electrons flow from carriers with more negative E0 (reduction potential) to carriers with more positive E0 As electrons transferred, energy released. 48 -Large difference in E0 (reduction potential) of NADH and E0 of O2 (1.14 Volt) -Hence large amount of energy released For ATP production Figure 10.9-ETC Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 49 ..ETC •to break down NADH and FADH2, pumping H+ into the outer compartment of the mitochondria . •In this reaction, the ETS creates a gradient which is used to produce ATP •Electron Transport Phosphorylation typically produces 32 ATP's •ATP is generated as H+ moves down its concentration gradient through a special enzyme called ATP Synthetase ….ETS Oxygen is the ultimate electron acceptor . As electrons are passed along chain, hydrogen (H+) atoms are pumped from the matrix to the inner membrane space of mitochondria. Exergonic energy created by electron flow powers H+ pumps. Metabolic water Metabolic water •Along with two electrons, at the end of the ETS each oxygen atom also is combined with two hydrogen ions •The net result is water (i.e., 2e- + 2H+ + ½O2 H2O) •This water is termed metabolic (as in, metabolic water) •Thus, one of the products of the complete oxidation of glucose is water & Energy Figure out How????? •ATP yield (36-38 ATP per molecule of glucose),How? 34 ATP produced by chemiosmotic synthesis (electron transport) 4 ATP produced directly: (2 ATP from glycolysis 2 ATP from citric acid cycle) 38 ATP theoretical maximum gross yield from the complete oxidation (-2 ATP required for active transport of NADH from glycolysis into the mitoch in some cells) 36-38 ATP net yield from one glucose molecule Oxidative Phosphorylation process by which ATP is synthesized as the result of electron transport driven by the oxidation of a chemical energy source Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 54 Oxidative phosphorylation •Oxidative phosphorylation is the phosphorylation of ADP using a mechanism powered by reduced electrons which, once their potential energy has been removed, are ultimately donated to atoms of oxygen •Substrate-level phosphorylation Substrate-level phosphorylation is the donation of a phosphate directly to ADP from a phosphorylated organic intermediate Phototrophy photosynthesis energy from light trapped and converted to chemical energy a two part process light reactions in which light energy is trapped and converted to chemical energy dark reactions in which the energy produced in the light reactions is used to reduce CO2 and synthesize cell constituents Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 56 Bibliography Lecture PowerPoints Prescott’s Principles of Microbiology-Mc Graw Hill Co. http://en.wikipedia.org/wiki/Scientific_metho d https://files.kennesaw.edu/faculty/jhendrix/bi o3340/home.html http://hyperphysics.phyastr.gsu.edu/Hbase/biology/atp.html http://www.uic.edu/classes/bios/bios100/l ecturesf04am/lect12.htm http://www.uic.edu/classes/bios/bios100/s ummer2003/krebsfull.htm http://www.nileshs.k12.il.us/jacnau/chpt9.html#Krebs%20 Cycle