* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chem 32 Solutions to Section 15.4 – 15.6 Homework Problems
Magnesium in biology wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Biosynthesis wikipedia , lookup
Photosynthesis wikipedia , lookup
Size-exclusion chromatography wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Mitochondrion wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Butyric acid wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Electron transport chain wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Microbial metabolism wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Biochemistry wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
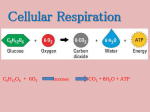
Chem 32 Solutions to Section 15.4 – 15.6 Homework Problems 15.78 Only pathway “c” produces energy that can be harnessed to make ATP. Pathway “a” does not produce or consume a significant amount of energy. Pathway “b” is an activation step, and consumes energy (the body breaks down ATP in this step). 15.86 The starting materials of the citric acid cycle are acetyl-CoA, ADP, phosphate, FAD, three molecules of NAD+, and three water molecules. The products are coenzyme A, ATP, FADH2, three molecules of NADH, three hydrogen ions, and two molecules of CO2. (Oxaloacetic acid is consumed in the first step, but is made in the last step.) 15.88 Milk spoils when certain bacteria carry out lactic acid fermentation. The product that gives the milk its “spoiled” aroma and flavor is lactic acid. 15.90 a) The starting materials of alcoholic fermentation are glucose, ADP, and phosphate ions. The products are ethanol, CO2, and ATP. b) Alcoholic fermentation produces two molecules of ATP for every molecule of glucose. 15.92 The total ATP yield from one pyruvate ion is 12.5 molecules of ATP. Here is how you can calculate this number. Oxidative decarboxylation (pyruvate → acetyl-CoA): 1 NADH = 2.5 ATP Citric acid cycle (acetyl-CoA → CO2): 1 ATP 3 NADH = 7.5 ATP 1 FADH2 = 1.5 ATP 15.96 The total ATP yield from one molecule of ethanol is 15 molecules of ATP. Here is how you can calculate this number. Ethanol → acetaldehyde: 1 NADH = 2.5 ATP Acetaldehyde → acetyl-CoA: 1 NADH = 2.5 ATP Citric acid cycle (acetyl-CoA → CO2): 1 ATP 3 NADH = 7.5 ATP 1 FADH2 = 1.5 ATP 15.98 a) The total ATP yield from three molecules of glucose is 80 molecules of ATP. 3 glucose → 5 pyruvate: 5 ATP 5 NADH = 12.5 ATP 5 pyruvate → 5 acetyl-CoA: (Each pyruvate gives us 1 NADH) 5 NADH = 12.5 ATP 5 acetyl-CoA → 10 CO2 (Each acetyl-CoA gives us 1 ATP, 3 NADH, 1 FADH2) 5 ATP 15 NADH = 37.5 ATP 5 FADH2 = 7.5 ATP b) For one molecule of glucose, the ATP yield is 80 ÷ 3 = 26.7 molecules of ATP. 15.102 a) The ATP yield from a molecule of capric acid is 64 molecules of ATP. The first step in the oxidation of any fatty acid is the activation step, which breaks down two molecules of ATP. Next, the fatty acid is broken down into acetyl-CoA. For a ten-carbon fatty acid, this requires four cycles of beta-oxidation, and the result is five molecules of acetylCoA. Finally, the acetyl-CoA is broken down to CO2 by the citric acid cycle. Activation step: –2 ATP Beta oxidation (4 cycles) 4 NADH = 10 ATP 4 FADH2 = 6 ATP Citric acid cycle (5 cycles) 5 ATP 15 NADH = 37.5 ATP 5 FADH2 = 7.5 ATP The overall total is 64 molecules of ATP. (Don’t forget to account for the two molecules that were broken down during the activation step.) b) Beta oxidation forms 16 molecules of ATP, as shown above. 15.118 The total ATP yield from one molecule of isoleucine is 33.5 molecules of ATP. isoleucine → acetyl-CoA (the reaction in the problem) 1 ATP 8 NADH = 20 ATP 3 FADH2 = 4.5 ATP acetyl-CoA → CO2 (citric acid cycle) 1 ATP 3 NADH = 7.5 ATP 1 FADH2 = 1.5 ATP converting NH4+ into urea –2 ATP Adding these up gives 33.5 molecules of ATP. 15.122 a) The yield is 8.3 moles of ATP per 100 g of asparagine (the calculator answer is 8.3255124 moles). The molecular formula of asparagine is C4H8N2O3, so the formula weight of asparagine is 132.124. The ATP yield per 100 g of asparagine is: 11 moles ATP 100 g × = 8.3 moles ATP 132.124 g asparagine €