* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Psychosocial Stress in Rats: Animal Model of PTSD Based on
Survey
Document related concepts
Aging brain wikipedia , lookup
Behavioral epigenetics wikipedia , lookup
Metastability in the brain wikipedia , lookup
Environmental enrichment wikipedia , lookup
Brain Rules wikipedia , lookup
Neuroeconomics wikipedia , lookup
Limbic system wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
State-dependent memory wikipedia , lookup
Holonomic brain theory wikipedia , lookup
Epigenetics in learning and memory wikipedia , lookup
Prenatal memory wikipedia , lookup
Social stress wikipedia , lookup
Transcript
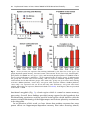
Psychosocial Stress in Rats: Animal Model of PTSD Based on Clinically Relevant Risk Factors 86 Phillip R. Zoladz and David M. Diamond Contents Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Behavioral, Physiological, and Neurological Aspects of PTSD . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Preclinical Models of PTSD . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Psychosocial Predator Stress Model of PTSD . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Recent Extensions of the Psychosocial Stress Model of PTSD . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Summary: The Challenge of Modeling PTSD in Animals . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Summary Points . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . References . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1533 1534 1535 1536 1543 1545 1546 1547 Abstract A primary goal of research on the neurobiological basis of post-traumatic stress disorder (PTSD) is to understand how emotional trauma can produce persistent detrimental effects on behavior and brain functioning. The goals of this area of research are to provide insight into biomarkers of emotional trauma and to develop more effective pharmacotherapy for PTSD. To accomplish these goals, P.R. Zoladz Department of Psychology, Sociology, and Criminal Justice, Ohio Northern University, Ada, OH, USA e-mail: [email protected] D.M. Diamond (*) Department of Psychology, Cognitive, Neural and Social Division, University of South Florida, Tampa, FL, USA James. A. Haley Veterans Hospital, Research and Development Service, Tampa, FL, USA e-mail: [email protected] # Springer International Publishing Switzerland (outside the USA) 2016 C.R. Martin et al. (eds.), Comprehensive Guide to Post-Traumatic Stress Disorders, DOI 10.1007/978-3-319-08359-9_58 1531 1532 P.R. Zoladz and D.M. Diamond the design of translational research needs to link animal models of PTSD to clinically relevant risk factors which address one’s resilience, as well as susceptibility, to develop persistent psychopathology in response to trauma. In the current review, we have discussed neurobiological and neuroendocrine aspects of PTSD and then briefly review the broad range of animal models of PTSD. We then discuss our psychosocial stress model of PTSD which is based on welldescribed PTSD-inducing risk factors, including a life-threatening experience, a sense of horror and uncontrollability, and an absence of social support and stability. Specifically, our psychosocial stress model integrates acute episodes of inescapable exposure of immobilized rats to a predator with chronic daily social instability. This stress regimen produces PTSD-like effects in rats at behavioral, cognitive, physiological, pharmacological, and genetic levels of analysis. Moreover, we also review our recent work that demonstrates greater evidence of neuroinflammation in rats exposed to the combination of psychological stress and physical (concussive) trauma. Overall, this translational approach has helped to bridge the gap between human and animal PTSD research and to create a framework for discovery of biomarkers of emotional trauma in clinical populations and toward the development of novel therapeutics for psychopathology. List of Abbreviations 5-HT ACTH BDNF BP CORT CRH EPM EPSP GABA GR HDAC HPA HR ICAM-1 LTP mTBI NE NF-L NMDA NOR PB PFC Serotonin Adrenocorticotropic hormone Brain-derived neurotrophic factor Blood pressure Corticosterone Corticotropin-releasing hormone Elevated plus maze Excitatory postsynaptic potential Gamma-aminobutyric acid Glucocorticoid receptor Histone deacetylase Hypothalamic-pituitary-adrenal axis Heart rate Intracellular cell adhesion molecule-1 Long-term potentiation mild traumatic brain injury Norepinephrine Neurofilament-L N-methyl-D-aspartate Novel object recognition Primed burst (potentiation) Prefrontal cortex 86 Psychosocial Stress in Rats: Animal Model of PTSD Based on Clinically. . . PSS PTSD SNS SSRI TIA VA 1533 Psychosocial stress (animal model of PTSD) Post-traumatic stress disorder Sympathetic nervous system Selective serotonin reuptake inhibitor Tianeptine Valproic acid Introduction Post-traumatic stress disorder (PTSD) is a unique psychiatric condition in that its diagnosis requires a distinct etiological event, specifically, one or more intense and horrific traumatic experiences. The individuals who develop PTSD following exposure to life-threatening trauma, such as wartime combat, motor vehicle accidents, or rape, typically endure chronic psychological distress by repeatedly reliving their trauma through intrusive, flashback memories (Reynolds and Brewin 1999). These individuals also develop an array of other debilitating symptoms, including persistent anxiety, an exaggerated startle response, cognitive impairments, and an impaired ability to extinguish conditioned fear (Stam 2007). Trauma exposure is a necessary, but not sufficient, component of PTSD development, expression, and most important, persistence. That is, only a subset (10–50 %) of traumatized individuals develops PTSD, depending on a multitude of interacting risk factors, including the nature of the trauma, genetics, gender, social support, and early life experiences (Zoladz and Diamond 2013). Thus, whereas most people exhibit PTSD-like symptoms as an early response to trauma, only a subset of traumatized people continue to experience symptomatology for 1–6 months, satisfying criteria for a diagnosis of chronic PTSD. Therefore, understanding susceptibility factors, such as peri-traumatic physiological responses, that promote persistent traumatic memory expression and PTSD symptoms, would be of great value. Indeed, researchers have shown that certain physiological responses to trauma, such as a blunted cortisol response or exaggerated sympathetic response, or the interaction between trauma and preexisting genetic vulnerabilities can predict a greater likelihood of PTSD development (Zoladz and Diamond 2013). Studies such as these are at the forefront of PTSD research because they can enable us to understand risk and resiliency factors associated with PTSD and therefore identify individuals who are at greater risk for developing the disorder. In this chapter, we have provided a brief overview of psychological and physiological factors involved in the susceptibility of a subset of individuals to express persistent PTSD symptoms. We have also described preclinical (animal) models of PTSD which have contributed to our understanding of how traumatic stress produces a lasting change in the brain and behavior. We then focused on our predator-based animal model of PTSD, as well as extensions of our model in work by other investigators, which may be of value toward the development of novel therapeutic treatments for PTSD. 1534 P.R. Zoladz and D.M. Diamond Behavioral, Physiological, and Neurological Aspects of PTSD PTSD is characterized by a complex aberrant biological profile involving several physiological systems, including the hypothalamus-pituitary-adrenal (HPA) axis and the sympathetic nervous system (SNS). Extensive work has reported abnormally low baseline levels of cortisol in PTSD which have been associated with the presence of enhanced negative feedback of the HPA axis and an elevated density of glucocorticoid receptors (Yehuda 2009). The reduced basal cortisol levels in PTSD do not appear to reflect adrenal insufficiency, as PTSD patients display robust HPA axis responsiveness, as evidenced by elevated cortisol levels in response to, and in anticipation of, acute laboratory stressors (Bremner et al. 2003; Elzinga et al. 2003). People with PTSD also demonstrate greater baseline and stress-induced elevations of SNS activity (Buckley and Kaloupek 2001), as measured by elevated baseline heart rate (HR), systolic blood pressure (BP), and diastolic BP, findings which resonate with research reporting an association between PTSD and increased risk for cardiovascular disease (Kubzansky et al. 2007). In response to traumatic reminders and standard laboratory stressors, people with PTSD display significantly greater increases in HR, BP, skin conductance, epinephrine, and norepinephrine than control subjects. Another indication of accentuated SNS activity in PTSD patients is the exaggerated responsiveness (i.e., greater increases in HR, greater increases in BP, greater expression of anxiety-like behavior) they exhibit following administration of yohimbine, an α2-adrenergic receptor antagonist that leads to increased central norepinephrine activity. These findings, along with those of greater baseline norepinephrine levels in PTSD patients, have implicated a major role for exaggerated noradrenergic activity in the hyperarousal component of PTSD (Strawn and Geracioti Jr 2008). Whereas numerous brain regions contribute to the behavioral and physiological manifestations of PTSD, much of the research has focused on the involvement of three primary structures, the amygdala, prefrontal cortex (PFC), and hippocampus. People with PTSD exhibit abnormal fear responses and diminished extinction of conditioned fear, which is suggestive of amygdala hyperactivity (Elzinga and Bremner 2002; Koenigs and Grafman 2009). Studies have also shown that PTSD patients have decreased PFC volume (Rauch et al. 2003; Woodward et al. 2006) and reduced activation of the PFC in response to the presentation of trauma-related, or fear-eliciting, stimuli (Lanius et al. 2001; Britton et al. 2005). PTSD patients are also impaired in tasks involving executive functioning, indicating impaired PFC functioning. Given the importance of the PFC in inhibiting amygdala-modulated emotional responses, reduced PFC activity in PTSD patients could promote amygdala hyperactivity, as well as the hypervigilance component of the disorder. Moreover, repeated activation of the traumatic memory, which would involve activation of the amygdala in conjunction with impaired PFC functioning, would contribute to traumatic memories to become even more intrusive and debilitating over time. Extensive work has also shown that people with PTSD have cognitive deficits which are suggestive of hippocampal dysfunction. This has been documented by reports of smaller hippocampal volume (Shin et al. 2006; Liberzon and Sripada 2008) 86 Psychosocial Stress in Rats: Animal Model of PTSD Based on Clinically. . . 1535 and impaired hippocampus-dependent learning and memory in PTSD patients (Gilbertson et al. 2001). However, close scrutiny of the literature suggests that severe deficits in hippocampal functioning with PTSD are actually uncommon, with numerous studies that have provided evidence of intact hippocampal functioning in PTSD. It appears that reduced hippocampal functioning may be a risk factor for, as well as an outcome of, PTSD (Zoladz and Diamond 2013). Preclinical Models of PTSD Whereas clinical research is vital for the implementation of novel treatments, animal models of PTSD provide a crucial complementary component to this process. In addition to their key role in establishing the safety and initial efficacy of novel therapeutic compounds, animal models are valuable in three key areas of treatment development. First, animal models facilitate the rapid cost-effective development of proof-of-concept studies to identify the most promising pharmacological candidates which can block trauma-induced behavioral and physiological abnormalities. This approach, with direct molecular assays of neural tissue, can improve our understanding of the mechanism of action of these compounds. Second, animal research provides for the assessment of the effects of interventions initiated prior to or soon after trauma occurs. This approach provides for the opportunity to develop preventive strategies which would be high risk, expensive, and potentially unethical to undertake in people. Finally, animal studies provide for the study of direct tests for different PTSD comorbidities and risk factors that might influence treatment responses in people, such as early life abuse, gender, social support, and traumatic brain injury. Preclinical research on traumatic stress has spawned a vast amount of research on the effects of exposing animals, primarily rodents, to strong stressful experiences followed by behavioral and physiological testing, which, in theory, provides insight into PTSD in traumatized people. However, there remain conceptual limitations to linking the study of stress in animals to generating a syndrome which resembles the clinical features of PTSD. For example, although people who experience a horrific event or rats that are exposed to a strong shock can have a strong memory of the trauma, the emotional memory of the experience, alone, does not represent the entire cluster of symptoms that define PTSD. Our view is that PTSD develops from the inability to cope with the memory of the trauma, but the memory, itself, is only one component of the entire syndrome. The challenge for an animal model of PTSD, therefore, is to not only generate a conditioned fear memory for a traumatic experience, but the trauma should produce behavioral and biological abnormalities which resemble those found in people diagnosed with PTSD. Researchers have used numerous different stressors to model aspects of PTSD in animals’ fear (Stam 2007), including electric shock (Servatius et al. 1995; Pynoos et al. 1996; Shimizu et al. 2004), underwater trauma (Richter-Levin 1998), stressrestress and single prolonged stress paradigms (Liberzon et al. 1997; Takahashi et al. 2006), and exposure to predators (Adamec et al. 2006; Wilson et al. 2013, 1536 P.R. Zoladz and D.M. Diamond 2014a, b, c) or predator-related cues (Cohen et al. 2006). The stressors employed in these studies typically produce physiological and behavioral signs of anxiety which persist beyond the time of the stress experience and in some cases, evidence of an exaggerated startle response, cognitive impairments, enhanced fear conditioning, resistance to fear extinction, and reduced social interaction. Although these studies have reported stress effects in animals which resemble those observed in people with PTSD, most have utilized only a small set of assessments, such as stress-induced changes in anxiety, without assessing the extensive abnormalities which make up the cluster of symptoms in PTSD. Moreover, many of the studies have evaluated stressinduced changes in responses for a relatively short period of time, without sufficient attention to the great persistence of PTSD symptoms. Finally, it is rare to find animal models of PTSD which assess the animal’s memory for the traumatic experience, itself. Often, the stress exposure is used only to disturb post-stress brain and behavior, instead of assessing the memory for the trauma experience, which is the hallmark feature of the PTSD syndrome. All of the approaches summarized above have contributed toward our understanding of how traumatic stress changes aspects of behavior and physiology, in general, and a subset specifically were designed to enhance our understanding of the neurobiology and endocrinology of PTSD. In the following section, we have discussed our psychosocial predator-based animal model of PTSD and how our findings may shed light on the development of novel pharmacotherapies for trauma, as well as neurobiological and endocrine abnormalities observed in people diagnosed with PTSD. Psychosocial Predator Stress Model of PTSD Pioneering research by the Blanchard group described how rats exhibit a strong innate fear of a predator, such as a cat (Blanchard et al. 1990). Further evidence of the effectiveness of predator exposure as a means with which to generate a fear response are findings in which predator exposure activates the HPA axis (Woodson et al. 2003; Masini et al. 2006; Park et al. 2008). At a functional level, extensive research demonstrates that predator exposure exerts a selective activation of brain circuitry (Silva et al. 2013) which may contribute to the profound capacity for predator exposure to impair spatial memory and synaptic plasticity in the hippocampus and to enhance synaptic plasticity in the amygdala (Diamond et al. 1999, 2006; Mesches et al. 1999; Woodson et al. 2003; Park et al. 2006, 2008; Vouimba et al. 2006; Vanelzakker et al. 2011; Zoladz et al. 2012b). For example, in one study, we demonstrated that a single acute (30 min) predator exposure occurring immediately after spatial learning impaired memory retrieval and also blocked the rapid phosphorylation of calcium/calmodulin-dependent protein kinase II (CaMKII), a critical component of the molecular basis of memory formation (Shonesy et al. 2014), in the dorsal CA1. It was intriguing that cat exposure, independent of whether it occurred in the context of blocking spatial memory or as an independent event, activated plasticity processes (increased phosphorylation of CaMKII) in the 86 Psychosocial Stress in Rats: Animal Model of PTSD Based on Clinically. . . 1537 Fig. 1 Acute (30 min) cat exposure after training (indicated by the black bar in the upper left graph) impaired spatial memory, increased serum corticosterone levels (lower left), blocked phosphorylation of CaMKII in CA1 (upper right), and increased phosphorylation of CaMKII in BLA (lower right). In all figures * indicates p < 0.05 compared to the “no stress: group. ** indicates that the group that received water maze training, and cat exposure exhibited significantly greater corticosterone levels than all other groups. The “train only” group was given water maze training to locate a hidden platform; “water only” was given water exposure without a hidden platform; “stress only” was exposed to the cat for 30 min only; and “train/stress” was given water maze training followed by cat exposure (Data from Zoladz et al. 2012b). In all figures, data are presented as the mean SEM basolateral amygdala (Fig. 1), a brain region which is central to trauma memory processing. Overall, these findings provided strong support for the hypothesis that life-threatening experiences activate specific brain regions which interfere with the memory-related functioning of the hippocampus and activate plasticity mechanisms in the amygdala. In an extension of this work, we have shown that predator exposure has more potent effects on hippocampus-dependent memory than other arousing stimuli, 1538 P.R. Zoladz and D.M. Diamond including footshock (Diamond et al. 2007), as well as exposure of male rats to a sexually receptive female rat (Woodson et al. 2003). Therefore, the ethological relevance and potency of predator exposure provides a well-established means with which to produce an intense, purely psychological, fear response which activates brain mechanisms of memory in rodent models of PTSD. Based on these observations regarding the instinctual fear generated in rats by cat exposure, our group developed a predator exposure-based animal model of PTSD. The primary components of the PTSD model were based on trauma-induction features that are known to be associated with a greater susceptibility of a subset of traumatized people to develop PTSD. Specifically, a subset of the DSM-V criteria for the diagnosis of PTSD includes the following three conditions: (1) PTSD can be triggered by an event that involves threatened death or a threat to one’s physical integrity; (2) a person’s response to the event involves intense fear, helplessness, or horror; and (3) in the aftermath of the trauma, the person feels as if the traumatic events were recurring, including a sense of reliving the experience. Therefore, in our work, rats are immobilized and placed in close proximity to a cat. As noted above, rats have an instinctual and intense fear of cats, which, in theory, would be intensified by their inability to escape (Maier and Watkins 2005). Although there is no physical contact between the rats and cat, the experience produces a profound physiological stress response in the rats, including elevated heart rate, blood pressure, and corticosterone levels (Zoladz et al. 2008a). A core symptom of PTSD is the repeated “reexperiencing” of the traumatic event that people with PTSD suffer from in response to activation of intense and intrusive memories of their trauma. For this reason, we included a “reexperiencing” component in our animal model of PTSD by giving rats the second cat exposure 10 days after the first (Zoladz et al. 2008a), which in theory would act upon a sensitized (hypertrophied) amygdala (Mitra et al. 2005). The second exposure of rats to the cat relates to work showing that PTSD develops in some people only after they have repeated traumatic experiences and work revealing that prolonged exposure to trauma increases the likelihood of developing symptoms of PTSD (Resnick et al. 1995; Gurvits et al. 1996). The manipulations described above all relate to the trauma memory, itself, but the PTSD syndrome is more than the response to the experience, alone. How one copes with trauma is a crucial component of PTSD susceptibility, which is expressed as the interaction among numerous factors, including trauma intensity and frequency, as well as intrinsic factors, such as one’s genetics, gender, early life experience, cognitive strategies, neurobiology, culture, and personality (Zoladz and Diamond 2013; Daskalakis et al. 2013). There is a vast literature on risk factors associated with the development of PTSD, with strong evidence to indicate that perceived social support can be a protective factor across a wide range of traumatic events. Conversely, insufficient social support and an unstable social life increase susceptibility to develop PTSD (Brewin et al. 2000; Ozer et al. 2003). Therefore, the final component of the PTSD model was daily social instability. Beginning with the day of the first cat exposure, the stressed rats were exposed to unstable housing conditions for the next 31 days. The rats were pair-housed, and every day, their cohort pair 86 Psychosocial Stress in Rats: Animal Model of PTSD Based on Clinically. . . 1539 Fig. 2 Timeline for the procedures employed in the psychosocial stress model of PTSD. Rats were placed in the fear conditioning chamber and then immobilized and exposed to the cat for 1 h on Days 1 and 11. Beginning on Day 1 and continuing throughout the 31-day stress period, the cohorts of rats in the cages were randomly changed, thereby providing daily social instability. Housing remained stable during all post-stress testing, which began on Day 32 combination was randomly changed. The social instability component was an integral component of the model, as we found that predator exposure, alone, did not produce persistent PTSD-like changes in the behavior of the rats (Zoladz et al. 2008a). The components and timeline of the PTSD model are illustrated in Fig. 2. Our psychosocial predator-based animal model of PTSD has produced numerous physiological and behavioral abnormalities which are remarkably similar to those observed in people with PTSD. For example, 3 weeks after the second predator exposure, rats exposed to our animal model of PTSD exhibit reduced growth rate, reduced thymus weight, greater adrenal gland weight, increased anxiety, an exaggerated startle response, impaired memory, greater cardiovascular and hormonal reactivity to an acute stressor, and an exaggerated physiological and behavioral response to the α2-adrenergic receptor antagonist, yohimbine (Zoladz et al. 2008a). An illustration of a subset of these PTSD-like effects, specifically the increase in anxiety, as measured by open arm time in the elevated plus maze, and the increase in startle response, is provided in Fig. 3. As mentioned earlier, a pathologically intense memory of the trauma is a hallmark feature of PTSD. Therefore, it was important to include a measure of the rat’s memory for the cat exposure experiences. To accomplish this goal, we measured the rat’s memory for trauma indirectly by placing the rat in a distinct chamber immediately prior to each of the two cat exposures (Zoladz et al. 2012a). The rats were left in the chamber for 3 min, and during the last 30 s of each exposure, they were presented with a tone. Then, they were removed from the chamber and immediately immobilized and given the 1 h cat exposure. The strategy behind this manipulation was to use a form of classical conditioning, in which the rats would form an association between the chamber and cue with the cat. This situation can be considered analogous to the panic response people with PTSD exhibit when they experience a cue, such as an odor or a sound, which reminds them of their traumatic 1540 P.R. Zoladz and D.M. Diamond Fig. 3 Effects of psychosocial stress on anxiety (left) as measured by open arm entries in the elevated plus maze and startle to a brief sound (right). Only the combination of cat exposure and social instability produce significant differences from the control group (Data from Zoladz et al. 2008) experience. Our test of the rat’s memory of the cat was confirmed with the finding that psychosocially stressed rats exhibited significant immobility (fear memoryinduced freezing) in response to being returned to the chamber, as well as the tone, that were both paired with the two cat exposures (Fig. 4). As discussed above, PTSD is also characterized by an aberrant biological profile in multiple physiological systems. One of the most extensively researched physiological systems in people with PTSD is the hypothalamic-pituitary-adrenal (HPA) axis. Empirical investigations of the adrenal hormone, cortisol, have reported abnormally low baseline levels of cortisol in PTSD patients. One explanation for the presence of low baseline cortisol levels in people with PTSD is that the disorder is associated with enhanced negative feedback inhibition of the HPA axis. Indeed, studies have reported that people with PTSD display an increased number and sensitivity of glucocorticoid receptors and an increased suppression of cortisol and adrenocorticotropic hormone (ACTH) following the administration of dexamethasone, a synthetic glucocorticoid (Yehuda et al. 2002, 2004; Duval et al. 2004). Studies have also employed the dexamethasone-corticotropin-releasing hormone (CRH) challenge paradigm to study abnormal HPA axis functioning in people with PTSD. Studies employing this paradigm have generally reported reduced ACTH levels in dexamethasone-treated PTSD patients who were subsequently administered CRH (Strohle et al. 2008). Therefore, we examined the effects of our animal model on rat corticosterone levels at baseline and following dexamethasone administration. We found that, at baseline, psychosocially stressed animals exhibited significantly 86 Psychosocial Stress in Rats: Animal Model of PTSD Based on Clinically. . . 1541 Fig. 4 Reexposure of the rat to the fear conditioning chamber on Day 32 generated a memorybased contextual fear (freezing) response (upper left), which is assumed to occur because the rat recalls the association of the chamber with the cat (illustrated in the red balloon; upper right). When the rat was placed in the novel chamber, the delivery of the tone reactivated the memory of the cat (lower right, thereby producing cue-induced freezing (lower left) (Data from Zoladz et al. 2012a) lower corticosterone levels than non-stressed rats. Following dexamethasone administration, psychosocially stressed rats displayed a blunted increase in corticosterone levels and a more rapid post-stressor recovery of those levels, relative to non-stressed control animals, following administration of an acute stressor (Zoladz et al. 2012a). These findings suggest that our PTSD regimen results in enhanced negative feedback of the HPA axis, as occurs in people with PTSD. One of the most promising areas of PTSD research is the study of the interaction of genetic and epigenetic factors with environmental stressors. Epigenetic alterations of the brain-derived neurotrophic factor (BDNF) gene have been linked to brain functioning, memory, stress, and neuropsychiatric disorders (Ikegame et al. 2013; Andero et al. 2014). Therefore, we examined whether there was a link between our 1542 P.R. Zoladz and D.M. Diamond Fig. 5 Levels of methylated bdnf exon IV DNA in the dorsal CA1 (dCA1), ventral CA1 (vCA1), prefrontal cortex (PFC), and basolateral amygdala (BLA) obtained on Day 32. Only the dCA1 exhibited a significant increase in methylated BDNF (Data from Roth et al. 2011) model and BDNF DNA methylation. We found that psychosocially stressed rats exhibited robust and selective hypermethylation of the BDNF gene in the dorsal CA1 of the hippocampus, with no evidence of methylation in the ventral CA1, amygdala, or prefrontal cortex (Fig. 5) (Roth et al. 2011). These results provide evidence that traumatic stress occurring in adulthood can induce CNS gene methylation and specifically support the hypothesis that epigenetic marking of the BDNF gene may underlie hippocampal dysfunction in response to traumatic stress. Furthermore, this work provides support for the speculative notion that altered hippocampal BDNF DNA methylation is a cellular mechanism underlying the persistent cognitive deficits which are prominent features of the pathophysiology of PTSD. Another goal of our animal model of PTSD was to assess whether pharmacotherapeutic agents can block the stress-induced abnormalities. We recently examined the effectiveness of amitriptyline (tricyclic antidepressant), clonidine 86 Psychosocial Stress in Rats: Animal Model of PTSD Based on Clinically. . . 1543 (noradrenergic antagonist), and tianeptine (glutamate modulator) in blocking physiological and behavioral sequelae manifested in our psychosocial predator stress model (Zoladz et al. 2013). Tianeptine, in particular, is important because it is a wellestablished and effective antidepressant (Brink et al. 2006; Kasper and McEwen 2008), which has been shown to block the adverse effects of stress on memory and brain functioning (Zoladz et al. 2008b; McEwen et al. 2010) and has been shown to produce salutary effects in the treatment of PTSD (Franciskovic et al. 2011). Treatment began 24 h after the first exposure of the rats to the cat, so as to mimic a clinically relevant condition in which people would initiate treatment within a day of a traumatic experience occurring. Whereas each of the drugs produced therapeutic effects on a subset of measures, tianeptine was the only agent to block the effects of chronic psychosocial stress in our entire battery of physiological and behavioral endpoints. Specifically, tianeptine blocked the expression of fear-conditioned memory in psychosocially stressed rats and also prevented the effects of psychosocial stress on anxiety, startle, cardiovascular reactivity, growth rate, adrenal gland weight, and thymus weight (Fig. 6). Importantly, these salutary effects of tianeptine occurred in the absence of adverse side effects. This finding may highlight the importance of initiating a treatment regimen as soon as possible after a person experiences trauma, as well as the potential value of tianeptine as a treatment for PTSD. Recent Extensions of the Psychosocial Stress Model of PTSD In work conducted in the past several years, our group has collaborated with investigators at Louisiana State University (LSU) and the Roskamp Institute in Sarasota, Florida, which has extended the clinical relevance of our psychosocial stress model of PTSD. The group at LSU, led by Joseph Francis and Brad Wilson, has conducted important studies on the effects of psychosocial predator stress on physiological measures, as well as effects of pharmacological treatments on brain neurotransmitter levels. They have demonstrated that psychosocial stress produces neurotransmitter changes which are similar to those seen in human patients with PTSD. Specifically, following 31 days of psychosocial stress, 5-HT decreased and NE increased in the hippocampus and PFC (Wilson et al. 2014a). In other works, these investigators demonstrated that psychosocial stress produced increased measures of oxidative stress and inflammation in the brain, adrenal glands, and systemic circulation (Wilson et al. 2013), factors which may play a critical role in the development of psychiatric, as well as somatic, symptoms of PTSD. Francis, Wilson, and their coworkers have also investigated the influence of daily treatment with valproic acid (VA), a histone deacetylase (HDAC) inhibitor, in the PTSD model. HDAC treatment is relevant to persistent PTSD effects as it can modify genetic transcription and diminish oxidative stress and levels of pro-inflammatory cytokines. They reported that VA attenuated the increase in oxidative stress and inflammation in the brain and blood produced by the PTSD manipulation (Wilson et al. 2014c). In more recent work, together we have found that 5-HT levels were normalized with chronic treatment of PTSD rats with a 1544 P.R. Zoladz and D.M. Diamond Fig. 6 Tianeptine treatment blocked cardiovascular effects, behavioral (anxiety) outcomes, and fear-conditioned memory effects produced by the psychosocial stress regimen. The tianeptinetreated group, unlike the vehicle (saline)-treated group, did not exhibit an increase in systolic/ diastolic BP (upper graphs), anxiety (EPM and startle; middle graphs), or fear memory to the context and cue associated with cat exposure (lower graphs) 86 Psychosocial Stress in Rats: Animal Model of PTSD Based on Clinically. . . 1545 selective serotonin reuptake inhibitor (SSRI) (Wilson et al. 2014b). Perhaps most important, NE levels remained significantly increased in response to rats administered the PTSD stress, as well as the drug, which may explain why sertraline provided no benefit in relation to anxiety or behavior in stressed rats (Wilson et al. 2014b). Thus, the relative ineffectiveness of SSRIs, in general, and sertraline, in particular, as a treatment for some forms of PTSD (Davis et al. 2001; Stein et al. 2002; Robb et al. 2010; Stoddard, Jr. et al. 2011) may occur because sertraline normalizes 5-HT levels, but the hypervigilance produced by elevated NE levels contributes to PTSD symptoms. Our PTSD model has also proven to be of value in the study of interactions between psychological stress and mild traumatic brain injury (mTBI). In this collaborative project, led by Fiona Crawford and Joseph Ojo at the Roskamp Institute, we reported that stress, composed of immobilization of mice exposed to predator (fox) odor, delivered in conjunction with mTBI, produced aberrant cognitive, behavioral, and neurochemical effects which were not observed with either mTBI or PTSD alone (Ojo et al. 2014). For example, the combination of stress and concussive head injury significantly impaired hippocampus-specific fear memory while leaving amygdala-based (cued) memory intact. This aberrant fear memory processing is highly relevant to the fragmented aspect of trauma memory which is the hallmark feature of PTSD (Van der Kolk and Fisler 1995; van Der et al. 2005). Moreover, indices of inflammation and neurodegeneration, such as a persistent increase in axonal injury and inflammatory markers (neurofilament-L (NF-L) and intracellular cell adhesion molecule-1(ICAM-1)), were greatest in the group that received psychological stress in conjunction with mTBI. These findings may be of value in addressing how mTBI occurring in combination with life-threatening emotional trauma produces a greater extent of neurological damage (Depue et al. 2014; Tanev et al. 2014). Summary: The Challenge of Modeling PTSD in Animals In this chapter, we addressed the challenge of translating stress research in animals to clinically relevant factors involved in PTSD. We have suggested that too often research on animals focuses on the stress response, itself, or fear conditioning, in isolation, thereby addressing only isolated components of PTSD etiology. We emphasized the importance of appreciating the range of clinically relevant features of PTSD, which includes the intrusive memory of the traumatic experience, in conjunction with risk factors, such as social support, and the chronic anxiety following trauma, because all components interact to influence whether or not a traumatized individual will develop PTSD. Hence, PTSD results from the impaired ability to cope with the traumatic experience and the memory it generated, rather than PTSD being composed of a strong memory of the trauma alone. 1546 P.R. Zoladz and D.M. Diamond We have taken these clinical risk factors into account in the development of our animal model of PTSD, which maximizes the likelihood of expression of stressinduced psychopathology in people. Specifically, we administered predator exposure to immobilized rats to provide them with a life-threatening experience in conjunction with an inability to escape. In theory, this experience to the rat is analogous to the PTSD induction feature involving a condition that evokes fear and horror in conjunction with a life-threatening experience. However, despite the fact that this experience provokes a powerful stress response in rats, as well as a fear-conditioned memory of the experience, cat exposure, alone, does not produce persistent PTSDlike abnormalities in behavior. It was only when we combined predator exposure with social instability that we observed persistent behavioral and physiological abnormalities in the rats which are similar to those found in people diagnosed with PTSD. Moreover, we have provided guidance for clinical research on PTSD with the finding of epigenetic modifications (methylation) of the BDNF gene in the hippocampus, which may provide the basis for impaired cognitive functioning in traumatized people. In addition, our work has identified the antidepressant tianeptine as a potentially useful pharmacological approach toward treatment of the cluster of symptoms found in PTSD. Finally, we have summarized recent work which has served as an extension of the original PTSD model by our collaborators Joseph Francis, Brad Wilson, and their coworkers at LSU and by Fiona Crawford, Joseph Ojo, and their coworkers at the Roskamp Institute. Each of these groups has provided important clinically relevant extensions of the original psychosocial stress PTSD model. The group at LSU has generated novel findings on neurotransmitter and neuroinflammatory abnormalities that develop in psychosocially stressed rats, with insight into how pharmacotherapies affect the brain and behavior. Our work with the group at the Roskamp Institute has provided evidence that psychosocial stress interacts with physical trauma to exacerbate brain inflammation and to produce PTSD-like cognitive abnormalities. Overall, integration of clinically relevant risk factors for people with PTSD with a reductionist approach in animal work provides an ideal strategy for generating translational research into the etiology and treatment of PTSD (Table 1). Summary Points • This chapter addressed three topics. First, we reviewed clinical research on the neurobiological factors involved in the susceptibility of a subset of traumatized individuals to develop PTSD. • Second, we provided an overview of animal models of PTSD. We consider all of this work to be of great value toward enhancing our understanding of the persistent effects of stress on the brain and behavior. However, we noted that there is a tendency for research programs to be narrowly focused on a small subset of stress effects without taking into account clinically relevant factors, such as persistent changes in behavior and physiology induced in a subset of traumatized individuals. 86 Psychosocial Stress in Rats: Animal Model of PTSD Based on Clinically. . . 1547 Table 1 Key facts in assessing animal models of PTSD PTSD is a unique psychiatric disorder in that its etiology necessarily involves exposure of an individual to one or more traumatic experiences. However, only a subset of traumatized people develops PTSD Animal models of PTSD need to take into account the trauma-induction feature of PTSD, as well as the differential susceptibility of a subset of individuals to develop psychopathology, which may result from hormone-gene-environment interactions In addition to the memory component of PTSD, there is a broad scope of symptoms in traumatized people, including altered expression of behavioral, physiological, and pharmacological measures that need to be taken into account in animal models It is important that an animal model of PTSD incorporate well-known risk factors, such as a lifethreatening experience and a lack of control and helplessness, both of which are commonly reported in the subset of traumatized people who develop PTSD An important factor in the differential development of PTSD is social support. Animal research that integrates chronic social components with acute life-threatening experiences will serve as more effective models of PTSD than models that focus on single factors, such as fear conditioning, alone This table lists key facts in assessing criteria and clinical relevance of animal models of PTSD. The key facts include the importance of including an assessment of traumatic memory, risk factors for a subset of individuals to develop psychopathology, and the expression of PTSD-like symptoms in traumatized animals • Third, we summarized our research on a psychosocial stress animal model of PTSD, which takes into account well-described risk factors for PTSD, including a horrific life-threatening experience, a lack of control, reexperiencing the trauma, social instability, and an unpredictable environment. • The combination of life-threatening experiences, immobilization, and social instability produced a remarkable PTSD-like syndrome in rats, including increased anxiety, an exaggerated startle response, impaired memory, greater cardiovascular and hormonal reactivity to an acute stressor, and exaggerated noradrenergic receptor sensitivity. In addition, rats administered the PTSD regimen exhibited BDNF gene methylation selectively in the hippocampus. • Psychosocial stress effects were completely blocked by daily treatment of rats with the antidepressant, tianeptine. These findings provide strong support for the suggestion that tianeptine may provide effective treatment for people with PTSD. • Collaborative work with other groups has demonstrated abnormal neurotransmitter and neuroinflammatory measures with the psychosocial stress model, as well as evidence of clinically relevant changes in neurotransmitter levels with SSRI treatment. In addition, the combination of psychological and physical stress produced a greater magnitude of neuroinflammation and cognitive abnormalities than by either manipulation alone. References Adamec RE, Blundell J, Burton P. Relationship of the predatory attack experience to neural plasticity, pCREB expression and neuroendocrine response. Neurosci Biobehav Rev. 2006;30:356–75. 1548 P.R. Zoladz and D.M. Diamond Andero R, Choi DC, Ressler KJ. BDNF-TrkB receptor regulation of distributed adult neural plasticity, memory formation, and psychiatric disorders. Prog Mol Biol Transl Sci. 2014;122:169–92. Blanchard RJ, Blanchard DC, Rodgers J, et al. The characterization and modelling of antipredator defensive behavior. Neurosci Biobehav Rev. 1990;14:463–72. Bremner JD, Vythilingam M, Vermetten E, et al. Cortisol response to a cognitive stress challenge in posttraumatic stress disorder (PTSD) related to childhood abuse. Psychoneuroendocrinology. 2003;28:733–50. Brewin CR, Andrews B, Valentine JD. Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. J Consult Clin Psychol. 2000;68:748–66. Brink CB, Harvey BH, Brand L. Tianeptine: a novel atypical antidepressant that may provide new insights into the biomolecular basis of depression. Recent Pat CNS Drug Discov. 2006;1:29–41. Britton JC, Phan KL, Taylor SF, et al. Corticolimbic blood flow in posttraumatic stress disorder during script-driven imagery. Biol Psychiatry. 2005;57:832–40. Buckley TC, Kaloupek DG. A meta-analytic examination of basal cardiovascular activity in posttraumatic stress disorder. Psychosom Med. 2001;63:585–94. Cohen H, Kaplan Z, Matar MA, et al. Anisomycin, a protein synthesis inhibitor, disrupts traumatic memory consolidation and attenuates posttraumatic stress response in rats. Biol Psychiatry. 2006;60:767–76. Daskalakis NP, Yehuda R, Diamond DM. Animal models in translational studies of PTSD. Psychoneuroendocrinology 2013;38:1895–1911. Davis LL, English BA, Ambrose SM, et al. Pharmacotherapy for post-traumatic stress disorder: a comprehensive review. Expert Opin Pharmacother. 2001;2:1583–95. Depue BE, Olson-Madden JH, Smolker HR, et al. Reduced amygdala volume is associated with deficits in inhibitory control: a voxel- and surface-based morphometric analysis of comorbid PTSD/mild TBI. Biomed Res Int. 2014;2014:691505. Diamond DM, Park CR, Heman KL, et al. Exposing rats to a predator impairs spatial working memory in the radial arm water maze. Hippocampus. 1999;9:542–52. Diamond DM, Campbell AM, Park CR, et al. Influence of predator stress on the consolidation versus retrieval of long-term spatial memory and hippocampal spinogenesis. Hippocampus. 2006;16:571–6. Diamond DM, Campbell AM, Park CR et al. The temporal dynamics model of emotional memory processing: a synthesis on the neurobiological basis of stress-induced amnesia, flashbulb and traumatic memories, and the Yerkes-Dodson Law. Neural Plast. 2007. 60803. Duval F, Crocq MA, Guillon MS, et al. Increased adrenocorticotropin suppression after dexamethasone administration in sexually abused adolescents with posttraumatic stress disorder. Ann N Y Acad Sci. 2004;1032:273–5. Elzinga BM, Bremner JD. Are the neural substrates of memory the final common pathway in posttraumatic stress disorder (PTSD)? J Affect Disord. 2002;70:1–17. Elzinga BM, Schmahl CG, Vermetten E, et al. Higher cortisol levels following exposure to traumatic reminders in abuse-related PTSD. Neuropsychopharmacology. 2003;28:1656–65. Franciskovic T, Sukovic Z, Janovic S, et al. Tianeptine in the combined treatment of combat related posttraumatic stress disorder. Psychiatr Danub. 2011;23:257–63. Gilbertson MW, Gurvits TV, Lasko NB, et al. Multivariate assessment of explicit memory function in combat veterans with posttraumatic stress disorder. J Trauma Stress. 2001;14:413–32. Gurvits TV, Shenton ME, Hokama H, et al. Magnetic resonance imaging study of hippocampal volume in chronic, combat-related posttraumatic stress disorder. Biol Psychiatry. 1996;40:1091–9. Ikegame T, Bundo M, Murata Y, et al. DNA methylation of the BDNF gene and its relevance to psychiatric disorders. J Hum Genet. 2013;58:434–8. Kasper S, McEwen BS. Neurobiological and clinical effects of the antidepressant tianeptine. CNS Drugs. 2008;22:15–26. Koenigs M, Grafman J. Posttraumatic stress disorder: the role of medial prefrontal cortex and amygdala. Neuroscientist. 2009;15:540–8. 86 Psychosocial Stress in Rats: Animal Model of PTSD Based on Clinically. . . 1549 Kubzansky LD, Koenen KC, Spiro III A, et al. Prospective study of posttraumatic stress disorder symptoms and coronary heart disease in the normative aging study. Arch Gen Psychiatry. 2007;64:109–16. Lanius RA, Williamson PC, Densmore M, et al. Neural correlates of traumatic memories in posttraumatic stress disorder: a functional MRI investigation. Am J Psychiatry. 2001;158:1920–2. Liberzon I, Sripada CS. The functional neuroanatomy of PTSD: a critical review. Prog Brain Res. 2008;167:151–69. Liberzon I, Krstov M, Young EA. Stress-restress: effects on ACTH and fast feedback. Psychoneuroendocrinology. 1997;22:443–53. Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev. 2005;29:829–41. Masini CV, Sauer S, White J, et al. Non-associative defensive responses of rats to ferret odor. Physiol Behav. 2006;87:72–81. McEwen BS, Chattarji S, Diamond DM, et al. The neurobiological properties of tianeptine (Stablon): from monoamine hypothesis to glutamatergic modulation. Mol Psychiatry. 2010;15:237–49. Mesches MH, Fleshner M, Heman KL, et al. Exposing rats to a predator blocks primed burst potentiation in the hippocampus in vitro. J Neurosci. 1999;19:RC18. Mitra R, Jadhav S, McEwen BS, et al. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc Natl Acad Sci U S A. 2005;102:9371–6. Ojo JO, Greenberg MB, Leary P, et al. Neurobehavioral, neuropathological and biochemical profiles in a novel mouse model of co-morbid post-traumatic stress disorder and mild traumatic brain injury. Front Behav Neurosci. 2014. doi:10.3389/fnbeh.2014.00213. Ozer EJ, Best SR, Lipsey TL, et al. Predictors of posttraumatic stress disorder and symptoms in adults: a meta-analysis. Psychol Bull. 2003;129:52–73. Park CR, Campbell AM, Woodson JC, et al. Permissive influence of stress in the expression of a U-shaped relationship between serum corticosterone levels and spatial memory errors in rats. Dose Response. 2006;4:55–74. Park CR, Zoladz PR, Conrad CD, et al. Acute predator stress impairs the consolidation and retrieval of hippocampus-dependent memory in male and female rats. Learn Mem. 2008;15:271–80. Pynoos RS, Ritzmann RF, Steinberg AM, et al. A behavioral animal model of posttraumatic stress disorder featuring repeated exposure to situational reminders. Biol Psychiatry. 1996;39:129–34. Rauch SL, Shin LM, Segal E, et al. Selectively reduced regional cortical volumes in post-traumatic stress disorder. NeuroReport. 2003;14:913–6. Resnick HS, Yehuda R, Pitman RK, et al. Effect of previous trauma on acute plasma cortisol level following rape. Am J Psychiatry. 1995;152:1675–7. Reynolds M, Brewin CR. Intrusive memories in depression and posttraumatic stress disorder. Behav Res Ther. 1999;37:201–15. Richter-Levin G. Acute and long-term behavioral correlates of underwater trauma– potential relevance to stress and post-stress syndromes. Psychiatry Res. 1998;79:73–83. Robb AS, Cueva JE, Sporn J, et al. Sertraline treatment of children and adolescents with posttraumatic stress disorder: a double-blind, placebo-controlled trial. J Child Adolesc Psychopharmacol. 2010;20:463–71. Roth TL, Zoladz PR, Sweatt JD, et al. Epigenetic modification of hippocampal Bdnf DNA in adult rats in an animal model of post-traumatic stress disorder. J Psychiatr Res. 2011;45:919–26. Servatius RJ, Ottenweller JE, Natelson BH. Delayed startle sensitization distinguishes rats exposed to one or three stress sessions: further evidence toward an animal model of PTSD. Biol Psychiatry. 1995;38:539–46. Shimizu K, Sawamura T, Nibuya M, et al. An animal model of posttraumatic stress disorder and its validity: effect of paroxetine on a PTSD model in rats. Nihon Shinkei Seishin Yakurigaku Zasshi. 2004;24:283–90. 1550 P.R. Zoladz and D.M. Diamond Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann N Y Acad Sci. 2006;1071:67–79. Shonesy BC, Jalan-Sakrikar N, Cavener VS, et al. CaMKII: a molecular substrate for synaptic plasticity and memory. Prog Mol Biol Transl Sci. 2014;122:61–87. Silva BA, Mattucci C, Krzywkowski P, et al. Independent hypothalamic circuits for social and predator fear. Nat Neurosci. 2013;16:1731–3. Stam R. PTSD and stress sensitisation: a tale of brain and body Part 1: human studies. Neurosci Biobehav Rev. 2007;31:530–57. Stein MB, Kline NA, Matloff JL. Adjunctive olanzapine for SSRI-resistant combat-related PTSD: a double-blind, placebo-controlled study. Am J Psychiatry. 2002;159:1777–9. Stoddard Jr FJ, Luthra R, Sorrentino EA, et al. A randomized controlled trial of sertraline to prevent posttraumatic stress disorder in burned children. J Child Adolesc Psychopharmacol. 2011;21:469–77. Strawn JR, Geracioti Jr TD. Noradrenergic dysfunction and the psychopharmacology of posttraumatic stress disorder. Depress Anxiety. 2008;25:260–71. Strohle A, Scheel M, Modell S et al. Blunted ACTH response to dexamethasone suppression-CRH stimulation in posttraumatic stress disorder. J Psychiatr Res. 2008;42:1185–1188. Takahashi T, Morinobu S, Iwamoto Y, et al. Effect of paroxetine on enhanced contextual fear induced by single prolonged stress in rats. Psychopharmacol (Berl). 2006;189:165–73. Tanev KS, Pentel KZ, Kredlow MA, et al. PTSD and TBI co-morbidity: scope, clinical presentation and treatment options. Brain Inj. 2014;28:261–70. Van der Kolk BA, Fisler R. Dissociation and the fragmentary nature of traumatic memories: overview and exploratory study. J Trauma Stress. 1995;8:505–25. van Der Hart O, Bolt H, Van der Kolk BA. Memory fragmentation in dissociative identity disorder. J Trauma Dissociation. 2005;6:55–70. Vanelzakker MB, Zoladz PR, Thompson VM, et al. Influence of pre-training predator stress on the expression of c-fos mRNA in the hippocampus, amygdala, and striatum following long-term spatial memory retrieval. Front Behav Neurosci. 2011;5:30. Vouimba RM, Munoz C, Diamond DM. Differential effects of predator stress and the antidepressant tianeptine on physiological plasticity in the hippocampus and basolateral amygdala. Stress. 2006;9:29–40. Wilson CB, McLaughlin LD, Nair A, et al. Inflammation and oxidative stress are elevated in the brain, blood, and adrenal glands during the progression of post-traumatic stress disorder in a predator exposure animal model. PLoS ONE. 2013;8:e76146. Wilson CB, Ebenezer PJ, McLaughlin LD, et al. Predator exposure/psychosocial stress animal model of post-traumatic stress disorder modulates neurotransmitters in the rat hippocampus and prefrontal cortex. PLoS ONE. 2014a;9:e89104. Wilson CB, McLaughlin LD, Ebenezer PJ, et al. Differential effects of sertraline in a predator exposure animal model of post-traumatic stress disorder. Front Behav Neurosci. 2014b;8:256. Wilson CB, McLaughlin LD, Ebenezer PJ, et al. Valproic acid effects in the hippocampus and prefrontal cortex in an animal model of post-traumatic stress disorder. Behav Brain Res. 2014c;268:72–80. Woodson JC, Macintosh D, Fleshner M, et al. Emotion-induced amnesia in rats: working memoryspecific impairment, corticosterone-memory correlation, and fear versus arousal effects on memory. Learn Mem. 2003;10:326–36. Woodward SH, Kaloupek DG, Streeter CC, et al. Decreased anterior cingulate volume in combatrelated PTSD. Biol Psychiatry. 2006;59:582–7. Yehuda R. Status of glucocorticoid alterations in post-traumatic stress disorder. Ann N Y Acad Sci. 2009;1179:56–69. Yehuda R, Halligan S, Grossman R, et al. The cortisol and glucocorticoid receptor response to low dose dexamethasone administration in aging combat veterans and holocaust survivors with and without posttraumatic stress disorder. Biol Psychiatry. 2002;52:393. 86 Psychosocial Stress in Rats: Animal Model of PTSD Based on Clinically. . . 1551 Yehuda R, Golier JA, Halligan SL, et al. The ACTH response to dexamethasone in PTSD. Am J Psychiatry. 2004;161:1397–403. Zoladz PR, Diamond DM. Current status on behavioral and biological markers of PTSD: a search for clarity in a conflicting literature. Neurosci Biobehav Rev. 2013;37:860–95. Zoladz PR, Conrad CD, Fleshner M, et al. Acute episodes of predator exposure in conjunction with chronic social instability as an animal model of post-traumatic stress disorder. Stress. 2008a;11:259–81. Zoladz PR, Park CR, Munoz C, et al. Tianeptine: an antidepressant with memory-protective properties. Curr Neuropharmacol. 2008b;6:311–21. Zoladz PR, Fleshner M, Diamond DM. Psychosocial animal model of PTSD produces a longlasting traumatic memory, an increase in general anxiety and PTSD-like glucocorticoid abnormalities. Psychoneuroendocrinology. 2012a;37:1531–45. Zoladz PR, Park CR, Halonen JD, et al. Differential expression of molecular markers of synaptic plasticity in the hippocampus, prefrontal cortex, and amygdala in response to spatial learning, predator exposure, and stress-induced amnesia. Hippocampus. 2012b;22:577–89. Zoladz PR, Fleshner M, Diamond DM. Differential effectiveness of tianeptine, clonidine and amitriptyline in blocking traumatic memory expression, anxiety and hypertension in an animal model of PTSD. Prog Neuropsychopharmacol Biol Psychiatry. 2013;44C:1–16.