* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download macromolecules
Biosequestration wikipedia , lookup
Nitrogen cycle wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Plant nutrition wikipedia , lookup
Proteolysis wikipedia , lookup
Photosynthesis wikipedia , lookup
Gaseous signaling molecules wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Microbial metabolism wikipedia , lookup
Biosynthesis wikipedia , lookup
Protein structure prediction wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
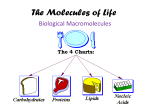
Chemistry of Life Unit Chapter 2-3 INTRODUCTION TO MACROMOLECULES MACROMOLECULES 1) 2) 3) “Macro” =???? Macromolecules are “giant molecules” Formed through polymerization a. The prefix “poly” refers to many b. Large compounds are formed by joining together smaller compounds c. Smaller units are called monomers d. Monomers join together to form larger polymers Small monomers may be identical or different Monomers are linked together like beads on a necklace to form the polymer MACROMOLECULES cont…. 4) Also known as Organic Molecules a. Contain the element carbon Can bond with many elements Including: Hydrogen, oxygen, phosphorus, sulfur and nitrogen Can bond with 4 elements at once Can form chains, rings, and complex structures b. Found in living organisms c. Sometimes called “biomolecules” MACROMOLECULES 4 Types of Macromolecules 1. Lipids 2. Carbohydrates 3. Proteins 4. Nucleic Acids Lipids Examples: Fats, oils, waxes & steroids Function: Waterproof coverings of cells, can be used for long-term energy storage. Elements: Mostly carbon and hydrogen with some oxygen. Monomer: 3 fatty-acids and 1 glycerol Polymer: Lipids – Unsaturated Fats: Liquid at room temperature due to double bonds (bent structure) – Saturated Fats: Solid at room temperature and no double bonds (full of hydrogen) Lipids Carbon Oxygen Hydrogen Long Chain Structure Smaller monomers linked to form a larger polymer Carbohydrates Examples: Sugars & Starches Function: Main source of energy in living things. Also structural purposes in plants. Elements: Carbon, hydrogen, and oxygen in a 1:2:1 ratio (ex. C6H12O6) Monomer: Monosaccharides – Simple sugars like glucose, fructose, and galactose Polymer: Polysaccharides (starches) – Cellulose: Plant starch – Glycogen: Animal Starch Carbon Carbohydrates Oxygen Hydrogen Ring Structure Smaller monomers linked to form a larger polymer Proteins Examples: Hair, nails and enzymes Function: Form muscles & bones, transport substances and control the rate of reactions in the body. Elements: Carbon, hydrogen, oxygen and nitrogen Monomer: Amino Acids Polymer: Protein Structure: Long chains of amino acids – 4 Levels of Structure Proteins Long Chain Structure Hydrogen Carbon Complex Folded Structures Oxygen Folded Structure Nitrogen Smaller monomers linked to form a larger polymer Nucleic Acids Examples: DNA & RNA Function: Store and transmit genetic information Elements: Carbon, hydrogen, oxygen, nitrogen and phosphorus Monomer: Nucleotide (sugar, phosphate & nitrogen base) Polymer: Nucleic Acid Structure: Long chains of nucleotides found in a twisted or folded structure Nucleic Acids Oxygen Nitrogen Ring Structure Carbon Hydrogen Folded Structure Smaller monomers linked to form a larger polymer VOCABULARY RECAP Macromolecules Carbohydrate Monomer Monosaccharide Polymer Polysaccharide Organic Lipid Fatty Acid Glycerol Unsaturated Fat Saturated Fat Cellulose Glycogen Protein Amino Acid Nucleic Acid Nucleotide