* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The Basics: A general review of molecular biology: DNA
Artificial gene synthesis wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Gene expression wikipedia , lookup
Expression vector wikipedia , lookup
Ribosomally synthesized and post-translationally modified peptides wikipedia , lookup
Magnesium transporter wikipedia , lookup
Interactome wikipedia , lookup
Ancestral sequence reconstruction wikipedia , lookup
Peptide synthesis wikipedia , lookup
Protein purification wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Western blot wikipedia , lookup
Homology modeling wikipedia , lookup
Point mutation wikipedia , lookup
Genetic code wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Metalloprotein wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Biosynthesis wikipedia , lookup
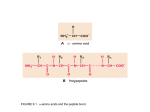
The Basics: A general review of molecular biology: DNA Transcription RNA Translation Proteins DNA (deoxy-ribonucleic acid) is the genetic material It is an informational super polymer -think of it as the blueprint DNA structure-- a polymer of nucleotides 1 Central dogma Protein synthesis: translation (mRNA to protein) Proteins have catalytic and structural functions Proteins with catalytic functions are enzymes 2 Proteins fold in an aqueous environment Life happens in water 70% of the cell is water Water is polar Water plays an important part in determining the final shape of proteins Water 3 How does it interact with other substances? Does it interact with other water molecules? If so, which atoms? Does it interact with Na+ (Blue) If so, which atoms? Does it interact with Cl- (Green) If so, which atoms? Does it interact with ethane If so, which atoms? Does it interact with ethanol? If so, which atoms? 4 http://www.3dchem.com/molecules.asp?ID=234 5 Amino acids are the building blocks of proteins: Common to all: Hydrogen atom Amino group Carboxyl group Distinguishing feature: R group; side chain conveys specific chemical properties Each table should construct two amino acids Short white bonds - hydrogens Thick gray bond - covalent bonds Long gray bond - covalent double bonds 6 Use MolyMod to have the students investigate the structure of the amino acid backbone and formation of a dipeptide 1. Examine the structure of 2 preassemble aa (side chains are green spheres) 2. Take aa apart and put them back together. 3. Join 2 aa to make a dipeptide, releasing water. 4. Join several dipeptides to make a longer peptide. 5. Examine structural constraints on the peptide chain -rotation of the phi (N-C) bond and psi (C-C) angles. Your structure should look like this 7 Protein synthesis--chain elongation mechanism Each table should make a dipeptide 8 Proteins also have a polarity to them-distinct beginning and end Free amino group At the first residue Free carboxy group at the last residue OH Fig 1-15 Different amino acids have different side chains file:///Volumes/AASK%2010-2006/Jmols/amino.html 9 Different amino acid chains have different chemical properties Activities for Amino Acid Side Chains and Properties 1. WSSP Amino Acid game: http://avery.rutgers.edu/WSSP/WSSPSummer06/WSSPSummer/AAGame/AAQuizPlusPenalty.php 2. Amino Acid matching game http://www.studystack.com/matching-23027 3. Amino Acid Quiz http://www.funtrivia.com/playquiz/quiz2422051bbb2a0.html 4. Asteroids AA game http://www.wiley.com/legacy/college/boyer/0470003790/animations/acideroids/acideroids.htm 5. Amino Acid Starter Kit (toobers with side chains) Helps sort the aa into different groups Using the chart, students mark the atoms in the side chains with stickers and sort the side chains. 10 Amino acid starter kit Use the key chart to arrange the amino acids on the circle Turn the circle around to look at the atoms of each side chain 11 Amino Acid Starter Kit (continued) Principals of protein folding 1. Have students choose 6 hydrophobic, 2 acidic, 2 basic, 2 cysteine, 1 methione and 2 poly side chains. 2. Mix and place aa along the toober. The aa order is the primary structure of the protein (Note: No two groups will get the same order). 3. Have the students fold the toobers using the following principals (not laws): 1. Hydrophobic aa should be hidden from polar water 2. Charged aa should be on the surface. 3. Positive and negative aa can be paired within one inch to neutralize the side chains 4. Polar side chains should be on the surface 5. Pair the cysteines to form a disulfide bond. Amino Acid Starter Kit (continued) Questions 1. Why should the Met be next to the blue cap? Proteins start with Met 2. As you added each new property what happened to the fold of the protein? The protein became more compact and complicated 3. Were you able to satisfy all the chemical properties? 4. Does your protein look like other students proteins? No, because they each have different aa sequences. 5. Given unlimited aa how many protein are there of 15 aa long? 2015 6. The average protein is 300 aa long. How many different proteins are possible? 20300 = 2 X 10390 7. How many proteins (genes) are in the human body? 50,000 (5 X 104) 8. What fraction of the total possible protein sequences are present in the human body? 2 X 10-386 9. Why are there so few? Relatively few sequences can satisfy all the chemical principals to form a stable shape. 12 Amino Acid Start Kit Variations: 1. Reversible Denaturation: a) b) c) Fold the protein and take a picture Unfold the protein then try to refold it. How does it compare to the original? 2. Reverse Engineering: Sometimes the protein sequence can not satisfy all the chemical principals. Try it in reverse a) b) c) d) Fold the toober into a compact shape without side chains Add the side chains so that they obey the chemical properties Unfold the toober and document the aa sequence. Are they similar to other groups? Unfold the protein 3. Effect of Mutations: Make changes in one of the hydrophobic side chains to a positive side chain What would happen to the protein fold? 13 Secondary Structure α-helices - right handed helix, 3.6 aa/turn, aa point outward stabilized by H-bonds between NH of aa X and CO of aa X+4 β-sheet - extended zig-zag structure parallel or antiparallel chains to form flat sheets aa side chains are above or below the sheets 14 a-helix and b-sheet construction kit 1. Assemble α-helix and β-sheet with components from the kit 2. What are the similarities and differences of the backbone aa? 3. Build an α-helix (10 aa) and 2 β-strands (5 each) a) How are these secondary structures the same or different? b) What does the effect of adding the H-bonds do to the structure? 4. Which atoms share these H-bonds? 5. Are these backbone or side chain atoms? 6. Measure the length of each structure Which is longer? Why? What implications does this have on protein folding? α-helix and β-sheet construction kit (cont.) 5. Organize the aa into groups and justify your groupings Nonpolar - Ala, Val, Leu, Ile, Phe, Polar -Gly, Ser, Thr, Asn, Gln, Trp, Tyr Postively charged - Arg, Lys, His Negatively charged - Asp, Glu Sulfur - Cys, Met 6. Why is there no Proline in the kit? 7. Assemble the side chains on the α-helix and β-strands How does the addition of these side chains affect its possible structure and interactions with water? 15 Use your assembled α-helix and add the amino acids side chains in the following order: N+- Glu-Glu-Lys-Ser-Ala-Val-Thr-Ala-Leu-Trp-Gly-CWhat effect to you think water has on folding this peptide? Sickle Cell Anemia is caused by a mutation in the β-globin gene of a Glutamate residue to Valine. N+- Glu-Glu-Lys-Ser-Ala-Val-Thr-Ala-Leu-Trp-Gly-CVal The mutation leads to deformed red blood cells resulting in anemia. What effect to you think this mutation has on folding this peptide? 16 Use your assembled β-strands and add the amino acids side chains in the following order: Strand#1 N+- Ile-Leu-Val-Glu-Leu -CStrand#2 C-- Ser-Val-Ser-Gly-Glu -N+ What differences do you see on one side of the sheet than the other? Examine secondary structure models with and without side chains What sides of these structures are likely to be in the interior of the protein? What sides of these structures are likely to be in the exterior of the protein? 17 Examine tertiary structure models of GFP and β-Globin Define the function of the proteins Identify and count the secondary structures Follow the chains through the entire protein. Are regions of the protein that are near each other on the primary sequence near each other in the tertiary structure? Zinc Finger Kit: Use toobers to form secondary structures and fold a protein 18 Form the β-strands Form the α-helix 19 Fold the secondary structures into the tertiary structure Zn Finger Kit Folding Questions 1. Describe the secondary structure elements that make up a Zn finger 2. How does the Zn atom stabilize the Zn finger motif? 3. Zn Finger proteins bind DNA. How might the arginine side chain in the model be involved in DNA binding? 4. How close is your model of the Zn finger to the model provided? 20