* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Polymers - Effingham County Schools
Survey
Document related concepts
Transcript
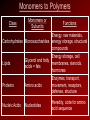
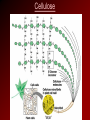
Glucose Molecule Macromolecules • Carbohydrates, proteins, and nucleic acids are polymers • Polymers – long molecules made from building blocks linked by covalent bonds • Monomers – the building blocks to polymers • Monomers are connected to form polymers by a condensation reaction, or specifically a dehydration synthesis, named because a water molecule is produced. • Polymers are broken down into monomers by hydrolysis, water is added to break the bonds. Dehydration Synthesis and Hydrolysis Dehydration reactions build macromolecules – enzymes facilitate this process Hydrolysis breaks down macromolecules; also with the aid of enzymes. Example – digestion of food Monomers to Polymers • Polymers of living things are made from only 40 to 50 common monomers • These monomers have the potential of producing thousands of different macromolecules • These macromolecules are responsible for the great diversity among individuals of a species and even greater diversity from species to species Monomers to Polymers Class Monomers or Subunits Functions Energy, raw materials, Carbohydrates Monosaccharides energy storage, structural compounds Energy storage, cell Glycerol and fatty Lipids membranes, steroids, acids = fats hormones Enzymes, transport, Proteins Amino acids movement, receptors, defense, structure Nucleic Acids Nucleotides Heredity, code for amino acid sequence Carbohydrates • Include sugars and their polymers • Monosaccharide – single sugar or simple sugar (ex. glucose) • Disaccharide – double sugar, formed by two monosaccharides bonded together (ex. sucrose – made from the monosaccharides glucose and fructose) • Polysaccharide – many monosaccharides bonded together (ex. starch – long chain of glucose molecules) Monosaccharides • Have the general molecular formula of (CH2O)n • Example: glucose (C6H12O6) a source of energy for cells • Name of monosaccharides usually ends in -ose • Have a carbonyl group and multiple hydroxyl groups • Carbon skeleton ranges from 3 to 7 carbons • The diversity of monosaccharides is due to the arrangements of parts around the carbons Monosaccharides Disaccharides • Joined by a glycosidic linkage, covalent bond b/w two mono. by a dehydration reaction Polysaccharides • Formed from a few hundred to a few thousand monosaccharides joined by a glycosidic linkage • Serve two main purposes: storage and structure Storage Polysaccharides • Starch – major storage polysaccharide in plants – Made up of glucose monomers – Source of stored energy – Humans and other animals hydrolyze the starch in potatoes and grains for a source of energy • Glycogen – storage polysaccharide in animals – Stored mainly in the liver and muscle cells – Reserves only last about 1 day in humans Structural Polysaccharides • Cellulose – Major component in plant cell walls – Made up of glucose monomers – Differs from starch in the linkage of the glucose monomers – Many animals, including humans, are unable to digest cellulose • Chitin – Makes up the exoskeletons of arthropods – Hardens with the aid of calcium carbonate – Also found in the cell walls of fungi Cellulose