* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Organic Molecules
Microbial metabolism wikipedia , lookup
Carbon sink wikipedia , lookup
Size-exclusion chromatography wikipedia , lookup
Metalloprotein wikipedia , lookup
Biosequestration wikipedia , lookup
Isotopic labeling wikipedia , lookup
Photosynthesis wikipedia , lookup
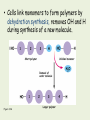
The Chemical Building Blocks of Life Organic Chemistry Comparison of Molecules • Inorganic – Chemistry of elements other than carbon • Organic – Carbon-based chemistry Inorganic •Usually with + & - ions •Usually ionic bonding •Always with few atoms • Often associated with nonliving matter • Organic •Always contain carbon and hydrogen •Always covalent bonding •Often quite large, with many atoms • Usually associated living systems • • All living things are mostly composed of 4 elements: C, H, O, N “CHON" • Compounds are broken down into 2 general categories: – Inorganic Compounds: • Do not contain carbon – Organic compounds • Contain significant amounts of carbon. • Often found with common "functional groups" … more on this in a minute! Why Carbon? • Carbon is essential to life for several reasons: – It can form strong stable (usually nonpolar) covalent bonds – It can form up to 4 chemical bonds due to 4 valence electrons – It can form multiple bonds More on the Carbon Atom • Carbon atoms: – Contain a total of 6 electrons – Often bonds with other carbon atoms to make hydrocarbons – Can produce long carbon chains like octane – Can produce ring forms like cyclohexane • Carbon skeletons vary in many ways Ethane Propane Carbon skeletons vary in length. Isobutane Butane Skeletons may be unbranched or branched. 1-Butene 2-Butene Skeletons may have double bonds, which can vary in location. Cyclohexane Skeletons may be arranged in rings. Benzene Figure 3.1, bottom part Functional groups help determine the properties of organic compounds • Functional groups – specific groups of atoms attached to carbon backbones • retain definite chemical properties • Functional groups are the groups of atoms that participate in chemical reactions – Ex. Hydroxyl groups are characteristic of alcohols – Ex. The carboxyl group acts as an acid Table 3.2 Macromolecules • Some molecules called macromolecules because of their large size • Usually consist of many repeating units – Resulting molecule is a polymer (many parts) – Repeating units are called monomers • Some examples: Category • Example • • Lipids • Fat • Glycerol & fatty acids • Carbohydrates • Polysaccharide • Monosaccharide • Proteins • Polypeptide • Amino acid • Nucleic Acids • DNA, RNA • Nucleotide • Subunit(s) Cells make a huge number of large molecules from a small set of smal molecules • Most of the large molecules in living things are macromolecules called polymers – Polymers are long chains of smaller molecular units called monomers (building blocks) – A huge number of different polymers can be made from a small number of monomers Dehydration and Hydrolysis • Dehydration - Removal of water molecule – Used to connect monomers together to make polymers – Polymerization of glucose monomers to make starch • Hydrolysis - Addition of water molecule – Used to disassemble polymers into monomer parts – Digestion of starch into glucose monomers • Specific enzymes required for each reaction – Accelerate reaction – Are not used in the reaction • Cells link monomers to form polymers by dehydration synthesis, removes OH and H during synthesis of a new molecule. 1 2 3 Short polymer Unlinked monomer Removal of water molecule 1 Figure 3.3A 2 3 Longer polymer 4 • Polymers are broken down to monomers by the reverse process, hydrolysis. (Breaks a covalent bond by adding OH and H.) 1 2 3 Addition of water molecule 1 Figure 3.3B 2 3 4 The Major Organic Groups What you need to know: • • • • The monomer of each group… The function of each group… The characteristics of each group… Unique properties of each group… Carbohydrates • Carbohydrates are loosely defined as molecules that contain carbon, hydrogen, and oxygen in a 1:2:1 ratio. – monosaccharides - simple sugars – disaccharides - two monosaccharides joined by a covalent bond – polysaccharides – made of many monosaccharide subunits Function • Primary Function: Quick Energy! Monosaccharides are the simplest carbohydrates • Monosaccharides are single-unit sugars • These molecules typically have a formula that is a multiple of CH2O • Hydrogen to oxygen ratio is 2:1. Hi Honey… I’m • Monosaccharides home! are the fuels for cellular work Figure 3.4A • The monosaccharides glucose and fructose are isomers – They contain the same atoms but in different arrangements Figure 3.4B Glucose Fructose Isomers • isomers - alternative forms of the same substance • ex. 8 isomers of “glucose” Cells link single sugars to form disaccharides • Monosaccharides can join to form disaccharides, such as sucrose (table sugar) Sucrose • and maltose (brewing sugar) • Which type of • reaction forms a disaccharide? Glucose Glucose Figure 3.5 Maltose • Polysaccharides are long chains of sugar units. These large molecules are polymers of hundreds or thousands of monosaccharides linked by dehydration synthesis. Starch granules in potato tuber cells Glycogen granules in muscle tissue Cellulose fibrils in a plant cell wall Cellulose molecules Figure 3.7 Glucose monomer STARCH GLYCOGEN CELLULOSE Storage polysaccharides…. • Starch - plant storage • Glycogen - animal storage – stored in the liver Electron micrograph of a section of a liver cell showing glycogen deposits as accumulations of electron dense particles (arrows). Mitochondria are also shown. x30,000. Structural Carbohydrates • Cellulose - plants – alpha form or beta form of ring – animals can not digest cellulose - “fiber” – most abundant form of living terrestrial biomass. • Chitin - exoskeleton of arthropods and in fungi cell walls – modified form of cellulose