* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download An introduction to the immune system: how vaccines work
Thiomersal controversy wikipedia , lookup
Lymphopoiesis wikipedia , lookup
Monoclonal antibody wikipedia , lookup
Social immunity wikipedia , lookup
Molecular mimicry wikipedia , lookup
Vaccination policy wikipedia , lookup
Immune system wikipedia , lookup
Hygiene hypothesis wikipedia , lookup
Adoptive cell transfer wikipedia , lookup
Adaptive immune system wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Innate immune system wikipedia , lookup
Herd immunity wikipedia , lookup
Whooping cough wikipedia , lookup
Childhood immunizations in the United States wikipedia , lookup
DNA vaccination wikipedia , lookup
Immunosuppressive drug wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
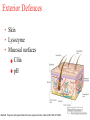
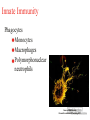
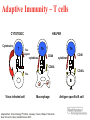
The Immune System and How Vaccination Works Rosalind Hollingsworth PhD Therapeutic Team Leader, Specialist Care Medical Department Agenda • Overview of the immune system • How vaccination works The Immune System (Aim: Recognise pathogen, eliminate it!) The ‘castle wall’ Exterior Defenses Innate Immunity The ‘soldiers’ Adaptive Immunity Exterior Defences • Skin • Lysozyme • Mucosal surfaces Cilia pH MacNeil. Progress and opportunities for tissue-engineered skin. Nature 2007;445: 874-880 Innate Immunity Phagocytes Monocytes Macrophages Polymorphonuclear neutrophils Source: MSN Encarta Dennis Krunkel/CNRI/Phototake NYC Phagocytosis in Action Adaptive Immunity • • • Lymphocytes 2 main categories T lymphocytes B lymphocytes Derived from bone marrow stem cells T cells develop in the thymus B cells develop in the bone marrow Lymphocytes • B lymphocytes produce antibody • T lymphocytes – 3 main groups: Control B lymphocytes and antibody production Seek and destroy virus-infected cells Assist phagocytic cells • ‘Key’ features: • Specific antigen recognition • Memory B Lymphocytes Bind specific antigen on surface receptors Divide and differentiate into plasma cells Produce large amounts of soluble receptor antibody http://www.nature.com/nature/journal/v421/n6921/fig_tab/nature01409_F1.html Antibodies (Immunoglobulins) Antibody Types Description IgA 2 Mucosal areas, such as gut, respiratory tract, urogenital tract. Prevents colonisation. Also found in saliva, breast milk and tears. IgD 1 Antigen receptor on B cells that have not been exposed to antigen. Function not well defined. IgE 1 Binds to allergens to trigger histamine release from mast cells involved in allergy. Also protects against parasitic worms. IgG 4 Provides the majority of antibodybased immunity from invading pathogens. IgM 1 Eliminates pathogens in the early stages of B cell mediated immunity before there is sufficient IgG. T Lymphocytes • T-helper cells (TH cells) Interact with mononuclear phagocytes Interact with B cells • T-cytotoxic cells (TC cells) • Kill virus-infected cells T Lymphocytes Adaptive Immunity – T cells CYTOTOXIC Cytotoxins T HELPER Th Fas Ligand cytokines Th CD40 cytokines CD40 CD40L CD40L Fas Virus infected cell Macrophage Adapted from: Immunobiology 5th Edition, Janeway, Travers, Walport, Shlomchik. New York and London, Garland Science 2001 Antigen specific B cell Adaptive Immunity – Primary and Secondary Responses IgG IgM/IgG IgM/IgG Taken from HPA National Immunisation Standards Slides http://www.hpa.org.uk/infections/topics_az/vaccination/slides.htm Agenda • Overview of the immune system • How vaccination works Immune Response to a Vaccine • Ideal vaccine - induce a similar or greater protective immune response as seen following natural infection • Stimulate antigen presenting cells to engage all aspects of immune response • Activate T and B cells to produce specific effector cells • Provide long-term protection through persistence of response and immunological memory Immune Response to a Vaccine Immunisation Challenge Immune response Protective threshold Duration Immune Response to a Vaccine • Example: Pneumococcal vaccination • Encapsulated bacteria • Protection correlates with production of anti-capsular polysaccharide antibody • Two types of vaccine: Streptococcus pneumoniae Plain polysaccharide (T independent) Polysaccharide conjugated to protein carrier (T dependent) Source: Carr J. CDC Public Health Image Library http://www.phil.cdc.gov/phil Immune response to a vaccine – Pneumococcal polysaccharide immunisation in adults 5 Immunisation 4 3 Antibody Concentration 2 1 0 0 1 12 36 months Ag B B B B B B B B B Data adapted from Sankilampi et al J. Infect. Dis 1997:176:1100-1104 B B Immune response to a vaccine – Pneumococcal polysaccharide-protein conjugate vaccine responses in children 14 antibody concentration (ug/mL) 12 Post 2 doses 10 8 Booster 6 4 2 0 Pre-Primary Post Primary Pre-Boost Post-Boost Ag DC Th B B B Th B B B B B Ag DC Th B B B B B B B B B B B B B B Goldblatt et al Pediatr Infect Dis J 2006 25: 312–319 Th B Th B Th B Th B B B Immune response to a vaccine – schedule of doses • 2, 3, 4 month schedule designed to give protection to infants as early as possible • One month gap: full response secondary response minimises potential for immune interference • Schedule may not be optimal for conjugate vaccines • Older infants respond better than younger infants • Interruption of immunisation series does not necessarily mean re-start the programme (Refer to Green Book recommendations) Immune response to a vaccine – Vaccine Failures • Primary failure – lack of an initial response to the vaccine which means the individual remains susceptible • Secondary failure – occurrence of disease despite an initial “protective” response to immunisation, correlates with waning immunity • A compromised immune system due to an underlying medical condition may lead to vaccine failure Department of Health “Immunisation Against Infectious Disease (The Green Book). Chapter 1 – Immunity and how vaccines work P4. Available online at: http://www.dh.gov.uk/en/Publichealth/Healthprotection/Immunisation/Greenbook/DH_4097254 Immune response to a vaccine – Vaccine Failure Immunisation Challenge Immune response Protective threshold Primary Vaccine failure Duration Secondary Vaccine failure Active Immunisation – Live Vaccines • Attenuated strains to ensure not pathogenic • Need to replicate to induce immune response Advantages Disadvantages Mimics natural infection Potential to revert to virulent strain Strong local and systemic responses Susceptible to interference with ongoing immune responses or passive antibodies One dose usually sufficient to induce long-lasting protection Contraindicated in immunosuppressed individuals Stability issues Potential for contamination Taken from HPA National Immunisation Standards Slides http://www.hpa.org.uk/infections/topics_az/vaccination/slides.htm Active Immunisation – Inactivated Vaccines • Suspensions of killed intact organisms – e.g. whole cell pertussis vaccine • Acellular and sub-unit vaccines – contain one or a few of the components (antigens) of the pathogen e.g acellular pertussis vaccine – contains 2-5 antigens of pertussis bacterium Taken from HPA National Immunisation Standards Slides http://www.hpa.org.uk/infections/topics_az/vaccination/slides.htm Active Immunisation - Inactivated Vaccines Advantages Disadvantages No potential for return to virulence Most require more than one dose Not susceptible to interference from ongoing immune responses/infections Shorter duration of immunity Good stability Local reactions common Constituents clearly defined – less chance of contamination Adjuvants required Taken from HPA National Immunisation Standards Slides http://www.hpa.org.uk/infections/topics_az/vaccination/slides.htm Additional Vaccine Components Component Purpose Example Adjuvants enhance the immune response to a vaccine aluminium salts Preservatives prevent bacterial or fungal contamination of vaccine thiomersal Additives stabilise vaccines from adverse conditions such as freeze-drying or heat, thereby maintaining a vaccine’s potency gelatine Residuals from manufacturing process Inactivating agents Antibiotics - prevent bacterial contamination during manufacturing process Egg proteins- some vaccine viruses are grown in chick embryo cells formaldehyde neomycin, streptomycin, polymyxin B influenza, yellow fever HepB vaccine Source: HPA minimum slide set for Core Curriculum for Immunisation Training Inactivated Vaccines – Conjugated Vaccines • Vaccines based on the capsular polysaccharide of bacteria poorly immunogenic in young children • The polysaccharide is bound (conjugated) to a protein carrier (eg diphtheria or tetanus toxoid) which enables the immune system of infants and young children to respond Conjugation Each saccharide is chemically activated Conjugation to carrier protein Combination Vaccines • Allows reduction in number of injections • Allows greater number of vaccines to be given at one time • Studies required to ensure safety and immunogenicity matches that of the individual vaccines Immunisation Schedule as of 20th April 2009 Age Vaccines 2 months DTaP/IPV/Hib + PCV7 3 months DTaP/IPV/Hib + MenC 4 months DTaP/IPV/Hib + PCV7+ MenC 12 months MenC/Hib 13 months MMR+PCV7 3 years 4 months – 5 years DTaP/IPV or dTaP/IPV + MMR Girls aged 12-13 years HPV 13 – 18 years Td/IPV Adapted from: Immunisation against Infectious Diseases, The Green Book. Available at: http://www.dh.gov.uk/en/Publichealth/Healthprotection/Immunisation/Greenbook/DH_4097254 Summary • Vaccination is the best known and the most successful application of immunological processes to human health • The ability of lymphocytes to develop memory is fundamental to this success Prevenar – Prescribing Considerations • Prevenar is contraindicated inpatients with a history of hypersensitivity to the active substances, to any of the excipients, or to diphtheria toxoid. • Prevenar will not protect against other Streptococcus pneumoniae serotypes than those included in the vaccine nor other micro-organisms. • The use of Prevenar does not replace the use of 23-valent pneumococcal polysaccharide vaccines in children 24 months of age with conditions placing them at higher risk for invasive disease due to Streptococcus pneumoniae. The interval between Prevenar and the 23-valent pneumococcal polysaccharide vaccine should not be less than 8 weeks. • As with other vaccines, the administration of Prevenar should be postponed in subjects suffering from acute moderate or severe febrile illness. • Prevenar should be administered intra-muscularly. • It is not recommended to administer Prevenar intramuscularly to infants or children with thrombocytopenia or other coagulation disorders. Please refer to official guidance for the administration of vaccines to children with these conditions. • Different injectable vaccines should always be given at different injection sites. • Common and very common adverse events include injection site reactions, drowsiness, fever, irritability, restless sleep, vomiting, diarrhoea, and decreased appetite. Prevenar – Abbreviated Prescribing Information Prevenar* Pneumococcal saccharide conjugated vaccine, adsorbed Presentation: Each 0.5ml dose of Prevenar contains 2 micrograms of each of the following saccharide serotypes: 4, 9V, 14, 18C, 19F, 23F and 4 micrograms of saccharide serotype 6B. Each saccharide is conjugated to the CRM197 carrier protein and adsorbed on aluminium phosphate. Indications: Immunisation against disease (including sepsis, meningitis, pneumonia, bacteraemia and acute otitis media) caused by Streptococcus pneumoniae serotypes 4, 6B, 9V, 14, 18C, 19F and 23F in infants and children from 2 months up to 5 years of age. Dosage and Administration: The immunisation schedules for Prevenar should be based on official recommendations. For intramuscular injection. Infants 2-6 months: Three doses, the first dose usually given at 2 months of age and with an interval of at least 1 month between doses. A fourth dose is recommended in the second year of life. Alternatively, when Prevenar is given as part of a routine infant immunisation programme, a two-dose schedule may be considered. The first dose may be given from the age of 2 months with a second dose at least 2 months later and a third (booster) dose at 11 – 15 months of age. Infants 7-11 months: Two doses with at least a 1 month interval between doses. A third dose is recommended in the second year of life. Children 12-23 months: Two doses with at least a 2 month interval between doses. Children 24 months-5 years: one single dose. The need for a booster dose after these immunisation schedules has not been established. Contra-indications: Hypersensitivity to any component of the vaccine or to diphtheria toxoid. Warnings and Precautions: Do not administer intravenously. Appropriate treatment must be available in case of anaphylaxis. The potential risk of apnoea and the need for respiratory monitoring for 48-72 hours should be considered when administering the primary immunisation series to very premature infants (born ≤ 28 weeks of gestation) and particularly for those with a previous history of respiratory immaturity. Impaired immune responsiveness may affect antibody levels. Prevenar does not replace 23-valent polysaccharide vaccine in at risk children 24 months of age. Children 24 months of age at high risk, previously immunised with Prevenar should receive 23-valent pneumococcal polysaccharide vaccine whenever recommended. Prophylactic antipyretics recommended when vaccinating children with history of seizure disorders, or when vaccinating simultaneously with whole cell pertussis vaccines. Delay vaccination in acute moderate or severe febrile illness. The immunogenicity of Prevenar has been demonstrated in infants with sickle cell disease. Safety and immunogenicity data are not yet available for children in other specific high-risk groups for invasive pneumococcal disease. Side Effects: Very common: Decreased appetite, vomiting, diarrhoea, injection site reactions (e.g. erythema, induration/swelling, pain/tenderness), fever equal to or over 38 °C, irritability, crying, drowsiness, restless sleep. Common: Injection site swelling/induration and erythema larger than 2.4cm, tenderness interfering with movement, fever over 39 °C. Uncommon: rash/urticaria. Rare: Seizures including febrile seizures, hypotonic hyporesponsive episode, injection site hypersensitivity reactions (e.g. dermatitis, pruritus, urticaria), hypersensitivity reactions including face oedema, angioneurotic oedema, dyspnoea, bronchospasm, anaphylactic/anaphylactoid reaction including shock. Very rare: Lymphadenopathy localised to the region of the injection site, erythema multiforme. Legal Category: POM Package Quantities: Pack of 1 single-dose pre-filled syringe (with separate needle) or pack of 10 single-dose pre-filled syringes Cost: Single-dose pre-filled syringe (with separate needle) pack of 1: £34.50. Single-dose pre-filled syringe pack of 10: £345.00 Marketing Authorisation Numbers: Single-dose pre-filled syringe (with separate needle) pack of 1:EU/1/00/167/006, single-dose pre-filled syringe pack of 10: EU/1/001/167/004 Marketing Authorisation Holder: Wyeth-Lederle Vaccines S.A., Rue du Bosquet 15,B-1348 Louvain-la-Neuve, Belgium For full prescribing information and details of other side effects see Summary of Product Characteristics Full prescribing information is available on request from: Wyeth Pharmaceuticals, Huntercombe Lane South, Taplow, Maidenhead, Berkshire, UK, SL6 0PH. Telephone: 0845 367 0098 Date of Prescribing Information: 06Jan09 Date of Preparation: 10Feb09 Code no. ZAPI104 Doc ID: 51558 *Trade mark