* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Presentation 2013-201307040352
Metalloprotein wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Microbial metabolism wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Metabolic network modelling wikipedia , lookup
Mitochondrial replacement therapy wikipedia , lookup
Pharmacometabolomics wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Mitochondrion wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Butyric acid wikipedia , lookup
Biosynthesis wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Biochemistry wikipedia , lookup
Citric acid cycle wikipedia , lookup
Basal metabolic rate wikipedia , lookup
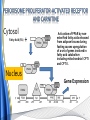
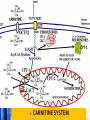
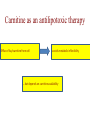
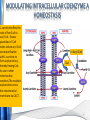
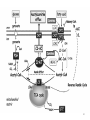
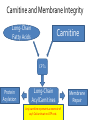
Carnitine: an overview Prof.Gianfranco Peluso M.D., Ph.D. Research Director IBP-National Research Council University of Piemonte Orientale 'A. Avogadro' 1 – Trimethylated aminoacid – Zwitterion – Sources: O CH3 + CH3 N CH3 CH2 CH OH - CH2 C O • Diet: red meats, dairies • Endogenous: protein catabolism Normally low Ratio AC/FC in plasma: 0.25 CH + 3 O R O CH3 N CH2 CH CH C 2 OCH 3 L-Carnitine a natural compound of the body Some basic characteristics of distribution: a Whole body stock (70 kilogram): approx. 20 gram Portion in skeletal muscle (30 kilogram): approx. 19 gram / 95% (3,9 µmol/g) a, b Portion in heart (300 gram): 0.25 gram (4,8 µmol/g) Portion in blood (5 Liter): 0.04 gram (0,05 µmol/ml) Fraction of Free L-Carnitine (FC): about 75 - 80 % c Fraction of Acyl-L-Carnitine (AC): about 20 - 25 % c a a,b Ratio AC / FC in plasma: < 0.25 c According to: a Scholte et al., 1987 b Opalka et al., 2001 c Boulat et al., 1993 Definition by medical dictionary CARNITINE Coenzyme of fatty acid oxidation and acetyl transfer; often designated vitamin BT, due to its vitamin role in Tenebrio sp. Present in high concentrations (5% dry weight) in meat extracts (latin carnis =meat). Tenebrio molitor TENEBRIO MOLITOR AND OBSERVATIONS ON THE EXPRESSION OF A CARNITINE DEFICIENCY by G. S. FRAENKEL -Department of Entomology, University of Illinois, Urbana 1958 Carnitine ● 75% provided in diet; 25% synthesized mainly in liver • meat, poultry, fish & dairy products (3 ounces beef steak=81 mg Carnitine; 3 ounces codfish=5mg Carnitine; 12 spears of asparagus =0.4mg Carnitine ) • Although in healthy subjects endogenous synthesis is adequate to maintain carnitine levels, addition of carnitine in the diet may be required during certain stages of the life cycle and in disorders, such as aging and diabetes. Thus, carnitine is considered a “conditionally essential” nutrient and the term “functional carnitine deficiency” has been proposed to define abnormal clinical presentations correctable by carnitine administration. L-Carnitine - The daily requirement Supply Biosynthesis: 15 – 20 mg / day (minimum requirement) Nutrition: 2 – 50; ~100 mg / day (meat) Elevated Requirement: Strenuous exercise, diseases, pregnancy, etc. Excretion Prevailing values: 15 – 60 - 120 mg/day Intensive exercise: > 120mg/day Balance Equalized: Not equalized: excretion = supply excretion > supply Consequence: L-Carnitine Deficiency In contrast to microorganisms (i.e., Pseudomonas sp., Acinetobacter sp., Enterobacteriacae) mammals lack the enzymes which are responsible for the degradation of carnitine Carnitine homeostasis in mammals is maintained by a combination of absorption of carnitine from dietary sources, a modest rate of endogenous synthesis, efficient reabsorption from the glomerular, and mechanisms present in most tissues that establish and maintain substantial concentration gradients between intracellular and extracellular carnitine pools. 8 TMA:trimethylamine FMO:flavin monooxygenases TMAO:trimethylamine-N-oxide Gut flora: Prevotella genus for carnivore Bacteroides genus for vegetarian 9 10 http://www.westonaprice.org/blogs/cmasterjohn/2013/04/10/does-carnitine-from-red-meatcontribute-to-heart-disease-through-intestinal-bacterial-metabolism-to-tmao/ 11 Carnitine: The Endogenous Synthesis ● 25% synthesized in different organs (The rate of carnitine biosynthesis in humans is estimated to be about 1.2 μmol per kg body weight per day) - liver (major site) - kidneys - brain O CH3 + CH3 N CH3 CH2 CH OH - CH2 C O Some proteins modify lysine to trimethyllysine using Sadenosylmethionine, (SAM) as the methyl donor to transfer methyl groups to the ε-amino of the lysine side chain. Hydrolysis of proteins containing trimethyllysine provides the substrate for the subsequent conversion to carnitine. Histone Calmodulin Myosin Actin Cytochrome c Proteolysis 15 Scheme of carnitine metabolism on the organism level with the indication of carnitine’ synthesis sites. The complex biological functions of Carnitine derive from the non-linear interactions of many gene products, interactions that are strongly modulated by the environment Carnitine system: A Tool for Understanding Functioning and Dysfunctioning Organs by System Biology 17 L-carnitine and acylcarnitine esters the enzymes and transport proteins required for their transport (included the plasma membrane carnitine carriers, OCTN) the carnitine biosynthesis pathway from lysine and methionine. 19 Nutrigenomics 20 The fate of fatty acyl CoA. Fatty Acid In the fed state, fatty acyl CoA is utilized for triglyceride synthesis, and fatty acid oxidation is dormant. With fasting, fatty acyl CoA is diverted into the oxidative pathway with a fall in conversion to triglycerides. 21 The carnitine system 22 Cytosol Fatty Acid (FA) Nucleus + Activation of PPAR by nonesterified fatty acids released from adipose tissues during fasting causes upregulation of a set of genes involved in fatty acid catabolism including mitochondrial CPT I and CPT II. Gene Expression 23 Since CPT are rate limiting for βoxidation of fatty acids, the upregulation of CPT might increase the demand of carnitine in cells with high rates of fatty acid oxidation. The upregulation of carnitine biosynthesis and OCTN2 by PPAR during fasting is a means to supply tissues with sufficient carnitine for metabolic requirement. Carnitine Carnitine The function One of the main functions of carnitine is: the transport of long-chain fatty acids into the mitochondrial matrix for β-oxidation to provide cellular energy 26 Organic Cationic Transporter Regulation of betaoxidation by plasmamembrane carnitine carrier 27 CPT-1 CACT CPT-1 CARNITINE SYSTEM 28 Learning carnitine function by carnitine deficiency diseases. 29 Carnitine deficiency can be characterized by low plasma and tissue carnitine concentrations and can be defined as a decrease of intracellular carnitine, leading to an accumulation of acyl-CoA esters and an inhibition of acyl-transport via the mitochondrial inner membrane. 30 Two forms: systemic carnitine deficiency with low carnitine levels in plasma and the affected tissues, and muscle carnitine deficiency, with low carnitine concentration restricted to muscle. Animal model: Juvenile Visceral Steatosis Mouse (severe lipid accumulation in the liver, hyperammonemia, hypoglycemia, cardiac hypertrophy, mitochondrial abnormalities in skeletal muscle and progressive growth retardation). Pathogenesis: Point mutation in the mouse homologue of OCTN2 carnitine carrier. 31 fatty metamorphosis of viscera due to OCTN2 mutation 32 33 Secondary carnitine deficiency: (e.g., genetically determined metabolic conditions, acquired medical conditions, or iatrogenic states). Increased renal loss of carnitine: Lowe syndrome, Cystinosis Lysinuric protein intolerance: >>Lysine in urine with <<Carnitine synthesis Cirrhosis or chronic renal failure: < carnitine biosynthesis Lacto-ovo–vegetarian diet Preterm neonates: impaired renal reabsorption of carnitine and immature carnitine biosynthesis Iatrogenic causes (i.e., Valproate therapy) Increased catabolism in patients with critical illness (i.e., cancer disease) Because milk is rich in lipids, it is presumed that carnitine is important in preparing the fetus for a postnatal milk diet. Carnitine carrier(s) is localized to the apical membrane of syncytiotrophoblasts (Lahjouji et al., 2004; Grube et al., 2005), and is responsible for delivery of carnitine to the fetus during development. During pregnancy, the use of certain anticonvulsants, able to inhibit carnitine uptake, carries a risk of fetal malformations, including congenital heart disease as well as lip and palatal deformity. It is hypothesized that interference of carrier-mediated carnitine uptake across placenta by antiseizure drugs increases the risk of fetal developmental defects. It is noteworthy that carnitine is an essential nutrient for newborn infants because the mechanism of its biosynthesis is not fully developed until later in postnatal development. The primary source of carnitine for a newborn is from the mother's milk (Sandor et al., 1982). The neonatal intestine has an avid carnitine absorption system of solute carriers .(Kwok et al., 2006). The carnitine produced in the mammary gland is excreted into the milk and then is absorbed in the baby's intestine. Mother's milk is the only source of carnitine for the newborn infant and is required for the β-oxidation of fatty acids. This process of carnitine transfer from mother to infant represents a coordinated mechanism of solute handling via carnitine transporters between individuals. ~1 in every 11 babies (8.23%) in Europe is born full-term with low birth weight (LBW) Global incidence of LBW ~17% 38 Maternal Low Protein Diet Full-term Low Birth Weight babies Fetal Programming Altered Physiology Altered Morphology Kidney; Liver; Gastrointestinal tract Altered absorption/production/excretion of carnitine •Hypertension •Hyperlipidemia •Diabetes Mellitus •Obesity Require altered optimal drug dosage for patient Altered Pharmacokinetics 39 Children who develop type 1 diabetes early in life have low levels of carnitine and amino acids at birth: does this finding shed light on the etiopathogenesis of the disease? M. Locatelli, et al. Conclusion: This is the first study demonstrating that children who develop T1D early in life showed reduced circulating carnitine and amino acid levels soon after birth. Their evaluation in the early neonatal period could represent an additional tool in the prediction of T1D and offer new strategies for possibly preventing the disease as early as from birth. 40 FA FA 43 FA 44 Why does carnitine deficiency affect so many organs? …. because the role of carnitine goes beyond the oxidation of fatty acids. 45 Carnitine and CoA axis • A decrease of carnitine Free CoA is required for: induces a decrease of matrix free CoA and a • the catabolism of parallel increase of the acyl several amino acids, CoA/CoASH ratio both of which are inhibitory for • for the detoxification of mitochondrial organic acids and dehydrogenases. • Consequently, not only the xenobiotics, oxidation of fatty acids,. • for pyruvate but also the utilization of carbohydrates, the dehydrogenase, catabolism of several • for α-ketoglutarate amino acids and the detoxification of organic dehydrogenase acids and xenobiotics 46 become impaired. • for Kreb’s cycle Disorders of the mitochondrial functions can cause severe impairment of fatty acid oxidation with accumulation of acyl-CoA intermediates. Transesterification with carnitine leads to the formation of acylcarnitine and the release of free CoA. These acylcarnitines are excreted readily in the urine with increased carnitine losses in the urine and systemic secondary depletion of carnitine. 47 48 Metabolic Flexibility the ability • to adapt substrate utilization to substrate availability • to switch between the oxidation of lipid as a fuel during fasting periods to the oxidation of carbohydrate during insulin stimulated periods 49 Substrate Competition Theory FASTING Randle cycle • byproducts of fatty acid βoxidation, including acetylCoA, and citrate, act as potent allosteric inhibitors of glycolysis and pyruvate dehydrogenase (PDH) 50 • Reverse Randle cycle FEEDING/INSULIN 51 Metabolic InFlexibility failure to shift from fatty acid to glucose oxidation during the transition • from fasting to feeding • from rest to vigorous exercise and vice versa 52 Myocardial metabolism in normal heart. Normal myocardium at resting state uses FFA as a preferred fuel source, which is energy rich substrate but yield less ATP per mol of O2. While in the state of increased peripheral physiological demand (e.g. exercise) myocardium uses glucose as energy source which is more efficient energy source and yield more ATP per mol of O2. ATP, Adenosine triphosphate; FFA, Free fatty acid; O2, oxygen; Pcr, Phospho creatinine 53 Myocardial Metabolism in Health The heart consumes more energy than any other organ and it is the greatest oxygen-consuming organ in the body, around 8–15 ml O2 min/100 g heart, with the capacity to increase up to 70 ml under exercise conditions. Everyday the heart beats about 100,000 times, pumps approximately 10 tons of blood through the body, and cycles about 6 kg of adenosine-triphosphate (ATP) (20– 30 times its own weight). Mitochondrial energy metabolism in heart failure: a question of balance Metabolic Demand Energy Metabolism • Heart must continually generate ATP • The heart has a very high energy at a high rate from mitochondrial demand to sustain contractile oxidative phosphorylation (>95%) , function, basal metabolic with the remainder being derived processes, and ionic homeostasis. from glycolysis and GTP formation in • The heart has a relatively low ATP the tricarboxylic acid (TCA) cycle. content (5 μmol/g wet wt) and high • Under normal conditions, there is rate of ATP hydrolysis (∼30 μmol·g complete turnover of the myocardial wet wt−1·min−1 at rest). ATP pool approximately every 10 s. • To sustain sufficient ATP • However, the adult heart normally obtains 60–80% of its ATP from fatty generation, the heart acts as an acid β-oxidation “omnivore” and can use a variety of different carbon substrates, (i.e., fatty acid, glucose, lactate, and ketone bodies), as energy sources. Cardiac energy substrate selection • In the fetal heart, glucose oxidation is favored, whereas FA oxidation serves as the major ATP-generating pathway in the adult myocardium. Hypertrophy and ischemia drive metabolism toward glucose utilization, whereas in uncontrolled diabetes, the heart utilizes FAs almost exclusively. In some cases, as in early response to pressure overload–induced hypertrophy, these metabolic shifts are thought to be protective. The regulation of myocardial metabolism is dependent on the availability and abundance of substrate, hormone levels, coronary blood flow and oxygenation and inotropic state of the tissue. With ageing, relative contribution of glucose as myocardial substrate increases as seen in elderly. Altered substrate supply and utilization by cardiac myocytes could be the initial trigger in the pathogenesis of CM. The shift towards increased fatty acids and decreased glucose utilization is linked to elevated circulating levels of FFA and triglycerides as a consequence of enhanced adipose tissue lipolysis, increased FFA uptake as well as high tissue FFAs caused by hydrolysis of augmented myocardial triglyceride stores. When FA uptake exceeds FA oxidation capabilities, lipid accumulation occurs resulting in lipotoxicity. 57 Various possible risk factors that could contribute to the development of Cardio-metabolic Cardiomyopathy 58 Mitochondrial dysfunction is at the basis of a constellation of metabolic abnormalities that contribute to chronic conditions and diseases 59 60 Skeletal muscle and heart contain over 95% of total body carnitine. Since skeletal muscle and heart lack the ability to synthesize carnitine, it is obvious that carnitine transport is fundamental to supply carnitine to these tissues. 61 Diet-induced and genetic forms of glucose intolerance were associated with high rates of incomplete fat oxidation (>>fatty acidderived acylcarnitine intermediates), which occurs when carbon flux through the βoxidation machinery outpaces entry of acetyl-CoA (the final product of β-oxidation) into the TCA cycle. Carnitine as an antilipotoxic therapy Efflux of Acylcarnitine from cell avoids metabolic inflexibility …but depends on carnitine availability 64 L-carnitine buffers the ratio of free CoA to acyl-CoA. Excess quantities of CoA esters induce acyl-CoA transesterification with L-carnitine to form acylcarnitines, thereby freeing CoA for use in other mitochondria reactions.The resultant acylcarnitines cross the mitochondrial membrane via CACT. >> Acyl-CoA CoA 65 Carnitine Acetyltransferase and Metabolic Flexibility 66 During the early phases of refeeding/reperfusion, heart mitochondria are confronted with a heavy influx of both glucose and fatty acid fuel. By permitting mitochondrial efflux of excess acetyl moieties, CrAT allows PDH activity to increase despite high rates of β-oxidation. This eases the Randle effect and facilitates a rapid transition from fatty acid to glucose oxidation. CrAT deficiency and/or carnitine insufficiency imposes a more rigid version of the Randle cycle, wherein feedinginduced stimulation of PDH activity is heavily dependent on the production of malonyl-CoA and resultant inhibition of CPT1. 67 68 Carnitine insufficiency results in abnormal fuel selection, evident in whole animals and in isolated mitochondria, and a corresponding decay in systemic glucose homeostasis. These disruptions in energy metabolism are associated with aberrant control of PDH activity. Carnitine mediates stimulation of PDH activity in heart mitochondria while also hampers the capacity of pyruvate to compete with and inhibit fatty acid oxidation. The therapeutic benefits of Carnitine supplementation corresponded with robust tissue efflux and urinary excretion of acetylcarnitine and acylcarnitines 69 Clinical Studies High doses of L-Carnitine in acute myocardial infarction: metabolic and antiarrhythmic effects. Rizzon et al, European Heart Journal 1989; 10: 502-508 Conclusions: •L-carnitine supplementation results in a significant increase in levels of carnitine esters in both the serum and urine. •The increased urinary excretion of long and short chain acylcarnitine esters induced by L-carnitine supplemetation may account for the reduction in ventricular arrhythmias observed in AMI patients. •L-Carnitine, by increasing the elimination of acylcarnitine esters, could therefore have a potential protective role on myocardial tissue, during AMI. Effects of L-Carnitine administration on Left Ventricular Remodeling after Acute Anterior Myocardial Infarction : the LCarnitine Ecocardiografia Digitalizzata Infarto Miocardico (CEDIM) Trial. Iliceto S et al Journal of the American College of Cardiology 1995; 2: 380-387 Conclusions: • The early and long-term administration of L-carnitine in patients with AMI is effective in attenuating progressive left ventricular dilation. • A still earlier administration of L-carnitine might exert a further protective effect on ischemia-reflow dysfunction within the risk area. Acute Myocardial Ischaemia Induces Cardiac Carnitine Release in Man Bartels GL., et al European Heart Journal 1997; 18: 84-90 Conclusions: • The moderate changes in carnitine metabolism during short periods of moderate myocardial ischemia may indicate cardiac Carnitine loss in situations under acute ischemic, metabolic stress. • This may indicate that L-Carnitine supplementation may be useful in patients with transient mild myocardial ischemia. Metabolic Treatment with L-Carnitine in Acute Anterior ST Segment Elevation Myocardial Infarction. Tarantini et al. Cardiology 2006; 106: 215-223 Conclusions: • The results of the study suggest that L-carnitine administration is particularly effective at reducing mortality when supplemented in the first week after anterior AMI. • The reduction in early mortality induced by L-carnitine treatment is likely due to the protection of the ischemic myocardium in the very acute phase of ischemia and reperfusion but could also be due to the protection of the acutely over-loaded non-infarcted region of the ventricle Three-year Survival of Patients with Heart Failure caused by Dilated Cardiomyopathy and L-Carnitine administration. Rizos I. American Heart Journal 2000; 139: S120-S123 Conclusions: • Three months treatment with 2g /day of oral L-carnitine improved survival of patients with moderate to severe heart failure due to dilated cardiomyopathy. • Three months L-carnitine treatment appears to improve the functional status of patients with moderate to severe heart failure attributable to dilated cardiomyopathy L-carnitine as an Adjunct Therapy to Percutaneous Coronary Intervention for Non-ST Elevation Myocardial Infarction Xue et al. Cardiovasc Drugs Ther (2007) 21:445–448 Treatment: • Carnitine 5 g IV bolus followed by 10 g/day IV infusion for 3 days Conclusions: • L-carnitine adjunct therapy appears to be associated with a reduced level of cardiac markers in patients with non-ST elevation acute coronary syndrome ) L-Carnitine prevents the development of ventricular fibrosis and heart failure with preserved ejection fraction in hypertensive heart disease Omori, et al J Hypertens, September 1, 2012; 30(9): 1834-44. Conclusions: Carnitine supplementation attenuates cardiac fibrosis by increasing prostacyclin production through arachidonic acid pathway, and may be a promising therapeutic option for HFpEF) Disturbed carnitine regulation in chronic heart failure Increased plasma levels of palmitoyl-carnitine are associated with poor prognosis Ueland et al. Int J Cardiol, May 21, 2012. Conclusions: Findings support a role for disturbed carnitine metabolism in the pathogenesis of HF, and suggest that some of its derivates could give prognostic information in these patients. Mitochondrial dysfunction is a common cause of peripheral neuropathy. Neuronal/axonal mitochondria impairment Peripheral neuropathy secondary to Schwann Cell mitochondrial dysfunction 79 Mitochondrial metabolic irregularities are a common culprit in diverse neurodegenerative diseases and are key pathological contributors to peripheral neuropathy. Mitochondrial dysfunction is thought to be largely responsible for the peripheral nerve deficits that afflict large numbers of people with diabetes and can lead to incapacitating pain, sensory loss, and debilitating muscle weakness. 80 Mitochondrial-dysfunction-induced shift in Schwann cells (SCs) lipid metabolism away from new lipid synthesis toward increased fatty acid oxidation. This metabolic alteration results in early depletion of myelin lipid components as well as a large accumulation of acylcarnitine (AC) lipid intermediates. Importantly, ACs are released from SCs and induce axonal degeneration. Activation of a maladaptive integrated stress response (ISR) and altered SC lipid metabolism resulting in toxic accumulation of lipid intermediates are thus underlying mechanisms of axonal degeneration and demyelination in mitochondrial peripheral neuropathies and constitute potentially important therapeutic targets. 81 tissue-specific deletion of the mitochondrial transcription factor A gene Lipid Classes CB: Cerebrosides ST: Sulfatides 82 Carnitine and Membrane Integrity Long-Chain Fatty Acids Carnitine CPT1 Protein Acylation Long-Chain AcylCarnitines Acyl-carnitine represents a reservoir of acyl-CoA units at no ATP cost. Membrane Repair