* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Toxic Effects of Nitric Oxide
Oxidative phosphorylation wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Lipid signaling wikipedia , lookup
Point mutation wikipedia , lookup
Biochemical cascade wikipedia , lookup
Biochemistry wikipedia , lookup
Paracrine signalling wikipedia , lookup
Endogenous retrovirus wikipedia , lookup
Western blot wikipedia , lookup
Expression vector wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Signal transduction wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Magnetotactic bacteria wikipedia , lookup
Metalloprotein wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Gaseous signaling molecules wikipedia , lookup
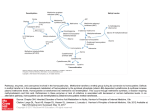
Toxic Effects of Nitric Oxide By Martha Gutierrez and Malerie Mock Free Radical Toxicity Free radicals are toxic to cells because of their reactivity with DNA, RNA, lipids, and proteins. Free radical cytotoxicity causes: Damage to cell membranes Disruption of cellular activities, such as cellular respiration and protein synthesis Host Defense and Toxicity Human phagocytic cells produce free radicals to inhibit infectious bacteria. Free radicals in high concentrations will exert toxic effects on all cells, including the cells that are producing the free radicals. Source: Fang, 2004 Nitric Oxide (NO) NO is a free radical that is employed by macrophages for defense. The toxicity of NO is attributed to its ability to bind to proteins that contain heme, iron, or copper, which will result in protein disruption. In reaction with a protein, NO can either be oxidized (lose electrons) or reduced (gain electrons). This ability is responsible for the high reactivity of free radicals. NO producing species are known as reactive nitrogen species (RNS). Source: Winstedt and Wachenfeldt, 2000 Reaction of NO Inside Host Cell NO reacts with superoxide inside a host cell to give a highly reactive intermediate product. NO ONOO- (peroxynitrite) ONOO- ONOOH (peroxynitrous acid) ONOOH is very unstable and a highly reactive oxidizing compound. ONOOH oxidizes nearby bio-molecules. Production of ONOOH is very rapid. Source: Rogstam et al., 2007 Reaction of Nitrosonium Ion (NO+) NO+ reacts with different organic side groups, especially thiols. Can also react with metals, lipids, and DNA. NO+ reactivity will damage cell membranes and shut down cellular activities. As a result of the high reactivity with a wide range of cellular components, NO+ is very toxic to cells. Source: Rogstam et al., 2007 NO+ Reaction NO+ reacts with thiols to yield S-nitroso compounds. Thiol containing peptides, amino acids, and proteins lose their biological activity. Cysteine contains a thiol group, and two cysteine residues form a disulfide bond. Cysteine thiol group replaced with S-nitroso disulfide bonds cannot be formed tertiary or quaternary protein structure is disrupted. Source: Rogstam et al., 2007 NO Production by Phagocytes In phagocytes, the ability to kill microorganisms is attributed to phagocyte-derived RNS. (Fang, 2004) In the cytoplasm, inducible NO synthase (iNOS) is a protein responsible for NO production in macrophages. Lipopolysaccharide production by bacteria stimulates NO production. NO is an immune response to pathogenic bacteria. (Rothfork et al., 2004) Inhibition by NO NO inactivates key enzymes that play an important role in microbial growth or infection, such as: Terminal respiratory oxidases Iron/sulfur protein aconitase NO reacts with Methionine to make it biologically inactive. Source: Ouellet et al., 2002 Terminal Respiratory Oxidase Terminal respiratory oxidase produces water molecules. If this enzyme is deactivated, then cell growth would be greatly reduced. Source: Ouellet et al., 2002 Iron-sulfur protein aconitase Aconitase is the enzyme that is responsible for the isomerization of citrate to isocitrate in the citric acid cycle. If inhibited by NO, the bacteria cannot produce ATP for energy. The citric acid cycle is inhibited by NO production by macrophages. HOONO Reaction with Methionine HOONO + Methionine sulfoxide + HONO HOONO is oxidized. Methionine sulfoxide is not biologically active. Source: Pryor et al., 1994 Methionine Inhibition of Staphylococcus aureus Auto inducing peptides (AIP) are secreted in large numbers by S. aureus. Under high AIP conditions, the bacteria upregulates the production of toxins. ONOOH inhibits AIP biologic function. Source: Rothfork et al., 2004 Inhibition of Staphylococcus aureus Inactivation of AIP is accomplished by the oxidation of the C-terminal methionine in the autoinducing peptide by ONOOH. Summary: OHOOH produced by macrophages Inactivates AIP from S. aureus Staphylococcal toxin production is not activated Decreased S. aureus virulence Source: Rothfork et al., 2004 How Does Bacteria Respond to NO Toxicity? Bacteria respond to NO toxicity by activating genes that encode for proteins that will detoxify NO, repair damage, and maintain homeostasis. Source: Justino et al., 2005 Escherichia coli Response to NO Toxicity E. coli uses NO reductase and flavohemoglobin, which plays a big role in RNS detoxification. Flavohemoglobins are enzymes that bind oxygen and reduce NO NO N2O NO NO3 Flavohemoglobins successfully render NO free radicals un-reactive. Source: Justino et al., 2005 Conclusion In macrophages, free radical production is important in innate immunity against numerous infections, such as S. aureus. E. coli has evolved a mechanism to detoxify and protect against NO. NO is extremely toxic to cells because it disrupts many cellular activities. Works Cited Fang, F. C. 2004. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nature Reviews Microbiology 2: 820-832. Justino, M., Vicente, J., Teixeira, M., and Saraiva, L. 2005. New Genes Implicated in the Protection of Anaerobically Grown Escherichia coli Against Nitric Oxide. Journal of Biological Chemistry 280: 2636-2643. Ouellet, H., Y. Ouellet, C. Richard, M. Labarre, B. Wittenberg, J. Wittenberg, and M. Guertin. 2002. Truncated hemoglobin HbN protects Mycobacterium bovis from nitric oxide. Proc. Natl. Acad. Sci. USA 99: 5902-5907. Pryor, W., Jin, X., and Squadrito, G. 1994. One and Two Electron Oxidations of Methionine by Peroxynitrite. PNAS 91: 11173-11177. Rogstam, A., Larsson, J. T., Kjelgaard, P., and von Washenfeldt, C. 2007. Mechanism of Adaptation to Nitrosative Stress in Bacillus subtilis. Journal of Bacteriology 189: 3063-3071. Rothfork, J., Timmins, G., Harris, M., Chen, X., Lusis, A., Otto, M., Ceung, A., and Gresham, H. 2004. Inactivation of Bacterial Virulence Pheromone by PhagocyteDerived Oxidants: New Role for the NADPH Oxidase in Host Defense. PNAS 101: 13867-13872. Winstedt, L. and Wachenfeldt, C. 2000. Terminal Oxidases of Bacillus subtilis strain 168: One Quinol Oxidase, Cytochrome aa3 or Cytochrome bd, Is Required for Aerobic Growth. Journal of Bacteriology 182: 6557-6564.