* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Aldehydes, Ketones and Carboxylic Acids
Cracking (chemistry) wikipedia , lookup
Metal carbonyl wikipedia , lookup
Elias James Corey wikipedia , lookup
Physical organic chemistry wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Carbohydrate wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Aldol reaction wikipedia , lookup
Hydroformylation wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
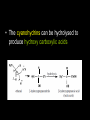
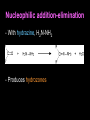
Aldehydes and Ketones AH Chemistry Unit 3(b) Functional Group • What is the functional group of the aldehydes and ketones? • What type of bonds link the C and O atoms? Physical Properties • How do the boiling points compare to alkanes? – Why? • How do the boiling points compare to alcohols? – Why? • Can aldehydes and ketones set up hydrogen bonds with each other? • Can they set them up with water? Chemical reactions • What are the characteristic reactions of aledehydes and ketones? – Reduction – Nucleophilic addition – Nucleophilic addition-elimination • Which is more susceptible to nucleophilic attack, aldehyde or ketone? – Why? Oxidation • What reagents can be used to oxidise aldehydes? Reduction • Aldehydes and ketones can be reduced to produce alcohols. • The reducing agent of choice is LiAlH4 - lithium aluminium hydride • Able to transfer a hydride ion to the partially positive carbon atom of the carbonyl group • Musty be carried out in anhydrous conditions (in ether) Nucleophilic addition • With HCN, producing cyanohydrins.... • The cyanohydrins can be hydrolysed to produce hydroxy carboxylic acids Nucleophilic addition-elimination - With hydrazine, H2N-NH2 - Produces hydrozones Nucleophilic addition Elimination CONDENSATION - With 2,4-dinitrophenylhydrazine - Produces 2,4-dinitrophenylhydrozones These products are “derivatives”. They are crystalline solids with characteristic melting points. The melting point of the derivative can be used to identify the orignial carbonyl compound.