* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapter 2: Genes in pedigrees
Point mutation wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
No-SCAR (Scarless Cas9 Assisted Recombineering) Genome Editing wikipedia , lookup
Nutriepigenomics wikipedia , lookup
Genetic engineering wikipedia , lookup
Cre-Lox recombination wikipedia , lookup
Ridge (biology) wikipedia , lookup
Genome evolution wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Dominance (genetics) wikipedia , lookup
Skewed X-inactivation wikipedia , lookup
Minimal genome wikipedia , lookup
Quantitative trait locus wikipedia , lookup
Gene expression programming wikipedia , lookup
Gene expression profiling wikipedia , lookup
Biology and consumer behaviour wikipedia , lookup
Y chromosome wikipedia , lookup
History of genetic engineering wikipedia , lookup
Neocentromere wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
Epigenetics of human development wikipedia , lookup
Genomic imprinting wikipedia , lookup
Designer baby wikipedia , lookup
Genome (book) wikipedia , lookup
Microevolution wikipedia , lookup
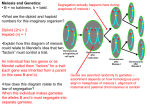
Chapter 2: Genes in pedigrees Haploid gametes are produced by meiosis Parents transmit their genes to their offspring via the gametes: the large immotile oocytes of the mother and the miniature motile spermatozoa of the father. Both types of gametes are produced by a specialized type of cell division, known as meiosis, that produces haploid gametes from diploid cells of the germ line. Contrary to the somatic cells and cells of the germ line, gametes only contain one copy of the “genomic encyclopedia”. The number of chromosomes found in haploid gametes is said to be “n”, while diploid cells contain “2n” chromosomes (corresponding to “n” pairs of homologous chromosomes or homologues). Before entering meiosis, spermatogonia in the testis and oogonia in the ovary undergo replication of their DNA during an S phase that is very similar to mitosis-‐preceding S-‐phases except for its slightly increased duration. After S-‐phase, each chromosome in the cell is present as two sister chromatids connected by cohesins. Gametogenesis then proceeds with a very unusual type of cell division: meiosis I. Rather than segregating one sister chromatid of each homologue in each of the two daughter cells, each cell will inherit the two sister chromatids of one homologue for each chromosome. As mitosis, meiosis I is subdivided in a prophase, metaphase, anaphase and telophase. Prophase I is itself subdivided in four stages: leptotene, zygotene, pachytene, and diplotene. Leptotene is characterized by the condensation of the replicated chromosomes and -‐ most strikingly -‐ by the pairing of homologues. The condensed sister chromatids of the paternal copy of chromosome 1 will align themselves with the homologous sister chromatids of the maternal copy of chromosome 1 along their entire length. The same will happen for each chromosome pair, forming as many bivalents. How are homologous chromosomes finding each other in the nucleus? Pairing appears to be mediated by the process of “homologous recombination”. A specialized protein (called Spo11 in yeast) introduces staggered cuts in the DNA known as double-‐ stranded breaks. On either side of the break, exonucleases then generate long, single-‐stranded 3’ overhangs. Proteins of the RecA family (called Rad51 in yeast) cover the single-‐strands and catalyze the search for homologous DNA sequences by strand-‐invasion, i.e. the formation of three-‐ stranded structures comprising a region of heteroduplex DNA and corresponding displaced strand. Branch migration extends the heteroduplex region, while formation of a double-‐ Holliday junction (involving DNA synthesis and ligation) seals the local connection between homologues. Pairing involves tens to hundreds of exchanges of this kind per chromosome pair. During zygotene, the alignment of homologous chromosomes is stabilized by formation of synaptonemal complexes: ladder-‐like structures comprising a pair of axial cores bridged by transverse filaments. Pachytene marks completion of synapsis. Double-‐Holliday junctions are resolved by formation of DNA strand cuts and re-‐ligation, which can happen in two ways. By far the most common pathway leaves a local segment of heteroduplex as sole trace of the recombination process. Approximately one double-‐Holliday junction per chromosome arm is resolved using a distinct pathway that creates a crossing-‐over between homologous sister chromatids. Crossing-‐ overs result in pairs of double-‐stranded-‐helixes with distinct parental origin on either side of the crossing over (maternal-‐CO-‐paternal and paternal-‐ CO-‐maternal). Paternal and maternal sequences may differ within the heteroduplex regions as a result of Single Nucleotide Polymorphisms (or SNPs) that occur in all populations (see hereafter). This may cause a mismatch that locally deform the DNA double helix and may trigger DNA repair. In this process, known as “gene conversion”, the sequence originating from one parent is “converted” to that of the other. Diplotene is characterized by the disassembly of the synaptonemal complexes, and further chromosomal condensation. Paired homologs remain connected at chiasmata (singular chiasma), the sites where crossing-‐overs occurred. The end of prophase I is referred to as diakinesis. The remainder of meiosis I (i.e. metaphase I, anaphase I, telophase I) is highly similar to mitosis, except that (i) the kinetochores of sister chromatids bind to microtubules originating from the same pole (rather than opposite poles as in mitosis), (ii) chiasmata connect homologues (rather than cohesins connecting sister chromatids during mitosis) prior to segregation, enabling alignment of the chromosomes on the metaphase plate, and (iii) cohesins at the kinetochores are protected from the action of separase by proteins called shugoshins. Meiosis II is in nearly all respect equivalent to a mitotic division. The diploid germ cells that undergo meiosis are called oogonia (singular oogonium) in females, and spermatogonia (singular spermatogonium) in males. Both types of cells derive from a population of Chapter 2: Genes in pedigrees Page 1/8 primordial germ cells (PGC) that migrate to the developing gonads. In a female fetus, PGC will divide mitotically in the 6 ovary to yield ∼5x10 oogonia. It is estimated that ∼22 cell divisions separate each oogonium from the zygote from which they derive. The fetal oogonia then enter meiosis I, which is – however -‐ arrested at the diplotene stage of prophase I. The corresponding germ cells are now called primary oocytes. Only at puberty, stimulated by gonadotropins secreted by the pituitary gland at each menstrual cycle, will one or a small number of primary oocytes complete meiosis I. The corresponding cytokinesis is strikingly asymmetric, yielding a large secondary oocyte inheriting virtually all the cytoplasmic content at the expense of the minute first polar body. Secondary oocytes are released from the ovary by the process of ovulation, yet remain arrested in metaphase II. Only if fertilized by a sperm cell, will secondary oocytes complete meiosis II, releasing a fertilized mature egg (or zygote) and second polar body. In a male fetus, PGC migrate in the developing 9 testis, where they multiply mitotically to yield ∼10 spermatogonia lining the basal membrane of the seminiferous tubules at the time of puberty. Each one of these is separated by ∼30 mitotic divisions from the zygote from which they derive. Starting at puberty, spermatogonia will engage every ∼two weeks in an asymmetric mitotic division, yielding (i) a stem cell-‐like spermatogonium to support future spermatogenesis, and (ii) a maturing spermatogonium that commits to completing spermatogenesis, implying three more mitotic divisions (yielding eight primary spermatocytes), followed by meiosis I (yielding sixteen secondary spermatocytes), and meiosis II (yielding 32 spermatids that will terminally differentiate in as many mature sperm cells). The 32 cells that derive from a maturing spermatogonium remain connected by cytoplasmic bridges, forming a syncytium until the very final stages of sperm maturation. Although genetically haploid, secondary spermatocytes and spermatids are in effect functionally diploid. As a result, although halve the secondary spermatocytes and spermatids are X-‐bearing and the other halve Y-‐bearing, all of them will contain all the gene products derived from the X and Y chromosome, of which some are essential to complete spermatogenesis. Contrary to the egg, which is separated from the zygote by a constant number of ∼23 cell divisions, the number of cell divisions leading from the zygote to a sperm cell equals ∼34 + (23 x number of years after puberty). Sexual reproduction, involving the fusion of haploid gametes contributed by animals of opposite sex, is an elaborate and costly process. Yet is utilized by the vast majority of plants and animals. It therefore must confer a selective advantage, which nature – however -‐ remains poorly understood. As a result of the independent segregation of the maternal and paternal homologues for distinct chromosomes (Mendel’s second law), as well as their reshuffling by the process of crossing-‐over, no two gametes produced by an individual are identical. For species producing many offspring, this might ensure that at least some offspring would be well adapted to a changing environment. Sexual reproduction promotes the combination of multiple favorable mutation having occurred in independent lineages, while asexual reproduction requires such favorable mutations to occur sequentially in the same lineage. Sexual reproduction has also been proposed as a means to eliminate deleterious mutations, which would otherwise accumulate in the population. In most sexually reproducing organisms, haploid cells do not multiply. Only the diploid somatic and germline cells multiply mitotically. In some primitive plants, diploid and haploid cells proliferate. In some species of yeast, only haploid cells proliferate, while the diploid zygote immediately engages in meiosis to produce new haploid cells. Mendel’s laws When asked what genetics is all about, students typically respond with “the science of heredity” or “the study of genes and their action”. Indeed as apparent from the table of contents of this course, genetics studies the behavior and mode of action of genes in cells (molecular genetics), pedigrees (factorial and quantitative genetics), and populations (population and evolutionary genetics). But, equally important, genetics also refers to a way of doing science, an experimental approach to unravel complex biological phenomena. If Gregor Mendel is as much revered by geneticists as he is today, it is because he was probably the first to apply this method, and this in an exemplary fashion. Providing extra appeal to geneticists, he applied it to the study of heredity. Mendel was born Johann in the now Czech Republic in 1822. He was raised on a farm, and went to University before entering priesthood and the Augustinian Abbey of St Thomas in Brno. He performed research in plant and animal breeding but also astronomy and meteorology. His now famous results on plant hybridizations went largely unnoticed for ∼35 years (including by Darwin) before being rediscovered around 1900. He is now generally considered to be the father of modern genetics. Mendel’s most famous experiments exemplify the key features of a well designed and executed experiment, and these are still highly relevant in the way science is conducted today. He first set the stage by carefully designing an experimental Chapter 2: Genes in pedigrees Page 2/8 F0: WW x ww F1: Ww F2 Male gametes W (1/2) w (1/2) Female gametes W (1/2) w (1/2) WW (1/4) Ww (1/4) Ww (1/4) Ww (1/4) The final but crucial stage was to test the proposed model. The model indeed makes a number of predictions, including about the expected phenotypic proportions on selfing F2, i.e. in the F3 generation. According to the model, wrinkled F2 are all homozygous ww and should therefore produce all wrinkled offspring when selfed. More interestingly, the model predicts that 1/3 of round F2’s should be homozygous WW and hence produce all round offspring only when selfed, while 2/3 of round F2’s should be heterozygous Ww and hence behave as the F2’s when selfed, i.e. yield ¾ round and ¼ wrinkled F3’s. And this is indeed what Mendel observed when he tested his hypothesis. Another important observation made by Mendel, was the “equivalence of reciprocal crosses”, i.e. the outcome was independent of the sex-‐by-‐ phenotype combination in the F0 generation. In the example of the seed shape, this means that the outcome of the crosses was the same whether the wrinkled F0 parent was male or female. In a second series of experiments, Mendel crossed pure-‐lines that differed for two binary phenotypes (f.i. round & yellow crossed with wrinkled & green). In agreement with the previous experiments, the F1 generation only exhibited the dominant phenotypes as defined before (f.i. round and yellow). When selfing the corresponding F1’s he always obtained the four possible phenotypic combinations in the following proportions in the F2: 9/16 (dominant-‐dominant; f.i. round-‐yellow), 3/16 (dominant-‐recessive; f.i. round-‐green), 3/16 (recessive-‐dominant; f.i. wrinkled-‐yellow), and 1/16 (recessive-‐recessive; f.i. wrinkled-‐green). The model he proposed to account for these observations, was that alleles of different genes segregate independently in the gametes (Mendel’s second law of independent segregation). Thus, if a individual is double heterozygote, he will produce four types of gametes in equal proportions. Applied to crosses between wrinkled-‐yellow and round-‐green peas, and referring to the dominant yellow alleles as G and to the recessive green allele as g, his model can be summarized as follows: F0: WWGG x wwgg F1: WwGg F2 Male Gametes system. In his case he used peas, which he could multiply in a controlled fashion. As peas are hermaphrodites (the same plant produces both male and female gametes), he could both “self” plants. But he could also perform outcrossing at will, by excising the undeveloped male pollen-‐ producing structures of a plant, and expose the female structures to mature pollen from a different plant using a pencil. Repeated selfing of plants with unique attributes (called phenotypes) let to pure lines, i.e. plants that would yield uniform offspring upon self-‐fertilization. By doing so Mendel developed pure “parental” lines (F0) that differed for one or more of seven “binary” phenotypes, including seed shape (round or wrinkled) and color (yellow or green). In his first experiments, Mendel crossed pure-‐lines differing for one such binary phenotype, producing a so-‐called F1 generation. He then selfed the F1’s, producing the F2 generation. The outcome of these crosses shared common features: (i) the F1’s exhibited the phenotype of one of the crossed parental lines, (ii) ¾ of the F2’s exhibited the same phenotype as the F1 generation, while the remaining ¼ re-‐expressed the phenotype of the F0 line unseen in the F1 generation. Mendel then proposed a model (Mendel’s first law of segregation) that would account for his observations. He posited that each binary trait is determined by a hereditary particle (now referred to as “gene”)(f.i. the gene determining seed shape), that comes in two forms or alleles (f.i. the round allele and the wrinkled allele). Individuals have two copies of each gene: one inherited from the father the other from the mother. Individuals inheriting the same allele from both parents are said to be homozygous or have homozygous genotype (f.i. wrinkled-‐wrinkled or round-‐round) and express the corresponding phenotype (respectively, wrinkled or round). Individuals that inherit a different copy from each parent are heterozygous (f.i. genotype round-‐wrinkled). As one allele is dominant (and the other recessive), heterozygous individuals express the same phenotype as the homozygotes for the dominant allele. Gametes contain only one copy of each gene. Gametes from heterozygous parents have equal probability to harbor either of the alleles of the parent. Referring to the dominant round allele as W and to the recessive wrinkled allele as w, Mendel’s model applied to crosses between pure lines of peas with round and wrinkled seeds can be summarized as follows: Female gametes WG (1/4) Wg (1/4) wG (1/4) wg (1/4) WG (1/4) WWGG (1/16) WWGg (1/16) WwGG (1/16) WwGg (1/16) Wg (1/4) WWGg (1/16) WWgg (1/16) WwGg (1/16) Wwgg (1/16) wG (1/4) WwGG (1/16) WwGg (1/16) wwGG (1/16) wwGg (1/16) wg (1/4) WwGg (1/16) Wwgg (1/16) wwGg (1/16) wwgg (1/16) Chapter 2: Genes in pedigrees Page 3/8 As before, this extended model makes a number of predictions which Mendel’s verified. One of the checks he carried out was to cross the F1’s with a tester. A tester is a pure line that is homozygous for the recessive alleles at all examined genes. As a consequence, the phenotype exhibited by the offspring directly reflects the genotype of the gametes transmitted by the F1. As predicted by his model, this test-‐cross yielded the four possible phenotypic combinations in equal proportions. is known, this can be indicated by writing the genotype as follows: AB/ab. Pairs of genes characterized by an excess of parental and lack of recombinant gametes are said to be genetically linked or exhibit genetic linkage. The proportion of recombinant gametes was rapidly shown to vary for different pairs of linked genes, and to be independent of the phase of the informative individual (i.e. it would be the same for an AB/ab and an Ab/aB individual). By performing an apparently simple “bean bag” experiment, and just on the basis of observed phenotypic proportions, Mendel proposed a model that accurately described essential features of heredity and meiosis. Make sure to identify the features of meiosis that underlie Mendel’s first law of segregation and second law of independent assortment. It is now well established that linkage occurs between genes that reside on the same chromosome. Such genes are said to be syntenic. The fact that Mendel did not observe linkage is due to the fact that -‐ by chance – the seven genes that he studied were non-‐syntenic. In the case of syntenic genes, recombinant gametes derive from meioses in which a crossing-‐over occurred between the two considered genes. Such event is more likely if the two syntenic genes are far apart than if they are close to each other. The degree of linkage, reflected by the proportion of recombinant gametes (or recombination frequency -‐ RF), can therefore be viewed as a measure of the distance separating syntenic genes. Subsequently, other scientists tested Mendel’s models, including in new model organisms such as the fruitfly D. melanogaster. In a few (lucky) instances, Mendel’s predictions proved to be inaccurate, and predictive models had to be amended accordingly. As an example, violation of the equivalence of reciprocal crosses let to the discovery of the sex chromosomes, and of Mendel’s second law to the discovery of genetic linkage. (Box Nomenclature) Genetic linkage Mendel’s second law states that an individual that is heterozygous for two genes (f.i. AaBb) produces four types of gametes in equal proportions: AB, Ab, aB and ab. However, geneticist discovered in the early 20-‐th century that Mendel’s second law didn’t apply to all pairs of genes. For such pairs, two types of gametes were significantly over-‐ represented at the expense of the two other classes. The over-‐represented types corresponded to the “parental” gametes, i.e. gametes with genotype identical to the ones inherited by the double heterozygous individual from its parents. Assume that the parents were respectively of AABB and aabb genotype, the informative AaBb individual would preferentially transmit “parental” AB and ab gametes, at the expense of so-‐called “recombinant” Ab and aB gametes. If – on the other hand – the parents were respectively of AAbb and aaBB genotype, the AaBb offspring would preferentially transmit “parental” Ab and aB gametes at the expense of “recombinant” AB and ab gametes. Determining which gametes transmitted by a double heterozygous individual are parental and which are recombinant is called “determining the linkage phase”. One way to deduce the linkage phase is from the analysis of the parental genotypes – as shown above. When the linkage phase of a double heterozygous individual It was quickly recognized that syntenic genes could be positioned relative to each other based on observed RF, i.e. that one could build genome maps by linkage analysis. Imagine that test-‐crossing an ABC/abc individual yields the following set of progeny: Phenotype Number Proportion ABC 350 .350 abc 360 .360 ABc 6 .006 abC 4 .004 Abc 92 .092 aBC 98 .098 AbC 43 .043 aBc 47 .047 Total 1,000 1 Remembering that in a testcross the phenotypes of the offspring directly reflect the genotype of the gamete transmitted by the triple heterozygote parent, the observed RF between A and B equals (92+98+43+47)/1000 or 0.28. A chi-‐squared-‐based goodness-‐of-‐fit test indicates that the observed gametic frequencies differ very significantly from those expected under Mendel’s second law, with an excess of parental over recombinant gametes typical of linked genes: O(bs) E(xp) 2 (O-‐E) /E AB ab Ab aB 350+6 250 360+4 250 92+43 250 98+47 250 44.9 52.0 52.9 44.1 2 Chi p 193.9 Chapter 2: Genes in pedigrees Page 4/8 -‐6 <10 The RF between A and C equals (6+4+92+98)/1000 or 0.20, which also departs very significantly -‐6 (pgoodness-‐of-‐fit<10 ) from Mendelian expectations indicating that A is also linked to C. Along the same lines, the RF between B and C equals -‐6 (6+4+43+47)/1000 or 0.10 (pgoodness-‐of-‐fit<10 ). The data thus indicate that A, B and C are syntenic, while analysis of pairwise RF (AB: 0.28; AC: 0.20; BC: 0.10) indicate that the correct gene order (out of the three possible orders) is A-‐C-‐B, i.e. that gene C is located between genes A and B. Note that we could have reached the same conclusions even without a priori (f.i. based on knowledge of the genotype of its parents) information about the linkage phase of the informative triple heterozygous parent. Indeed, the two most frequently transmitted gametes (ABC: 350/1000 and abc: 360/1000) indirectly determine the phase, while the two least common gametes (ABc: 6/1000 and abC: 4/1000) identify the double recombinants thereby unambiguously establishing order. It is worthwhile noting that the distances in RF-‐ units between A-‐C (0.20) and C-‐B (0.10) add up to more than the distance between A-‐B (0.28). This non-‐additivity is of course not a desirable property for a distance measure. Closer examination indicates that the lower than expected RF between A and B is due to the fact that 10 gametes have been counted as parentals, while being in fact double-‐recombinants (having recombined in both the A-‐C and C-‐B intervals). The fact that we have information about C between A and B allowed us to detect recombinational events, which we would have missed otherwise. If, rather than considering these 10 gametes as parental, we consider that they jointly contribute 20 recombination events, the corrected RF between A and B becomes 0.30 which is exactly equal to 0.20 (A-‐C) + 0.10 (C-‐B). This indicates that a distance measure based on the number of CO in the considered interval would be better than one based on the observed proportion of parental gametes (which in fact includes gametes with zero but also with an even number of CO in the interval of interest). As a matter of fact, even if one can’t directly observe all CO that may have occurred between two loci from genotype data, one can fairly accurately estimate their frequency from the observed RF. The mathematical relationship between the observed RF and the estimated CO frequency (COF) is called a mapping function. The simplest mapping function is Haldane’s. It can be derived as follows. Assume two syntenic loci, A and B. Assume the many meioses occurring in the gonads of a double heterozygous AB/ab individual. A proportion of meiosis will be characterized by 0 chiasmata (CH) between A and B, a proportion by 1 CH, a proportion by 2 CH, a proportion by 3 CH, etc ... We will assume that these proportions are accurately described by a Poisson distribution, i.e. p(x;m) = e−m m x x! in which m is the average number of CH occurring between A and B across all meioses. Note that the -‐m proportion of meiosis with 0 CH hence equals e . By definition gametes issued from meiosis with 0 CH between A and B can only be parental. Gametes issued from meioses with one CH between A and B, have 50% chance to be recombinant and 50% chance to be parental. This is due to the fact that CH occur at prophase I, involving two of the four present chromatids. Paradoxically, gametes issued from meiosis with two or more CH between A and B, likewise have 50% chance to be recombinant and 50% chance to be parental. This can be seen for meiosis with two CH as follows. Let us label the sister chromatids on the paternal homologue 1 and 2, and those on the maternal homologue 3 and 4. Each CH can involve four possible chromatid combinations (1+3; 1+4; 2+3; 2+4). Meioses with two CH can thus correspond to 16 possible chromatic combinations, which are a priori equally probable. For four of these, the two CH involve the same chromatid pair (f.i. 1+3 & 1+3). All gametes derived from such meiosis are parental. For eight, the two CH involve three chromatids (f.i. 1+3 & 1+4). Halve the gametes derived from such a meiosis will be parental, the other halve recombinant. For the remaining four, the two CH jointly involve the four chromatids (f.i. 1+3 & 2+4). All gametes derived from such meiosis will be recombinant. Taken together, half the gametes derived from meioses with two CH between A and B will be parental, the other half recombinant. The same conclusion can be reached for meioses with three CH between A and B (64 possible chromatid combinations), four CH, etc. Therefore, the expected RF between loci A and B equals: 1 RFA−B = (1− e−m ) 2 where (1− e−m ) corresponds to the proportion of meiosis with at least one CH between A and B. Geneticists prefer to use the average number of CO (COF) per gamete as distance measure, rather than the average number of CH per meiosis (m), but these two are simply related as 2xCOF=m. Hence, 1 RF = (1− e−2*COF ) 2 and Chapter 2: Genes in pedigrees Page 5/8 1 COF = − ln(1− 2RF) 2 COF is expressed in morgans or centimorgans (cM). One cM corresponds to one CO per hundred gametes. Thus RF between loci are measured from the genotype data, and subsequently converted to cM (i.e. an additive measure of genetic distance) using mapping functions. Haldane’s mapping function assumes that CH occur at random along a bivalent and have no influence on each other. This does not correspond to the reality. First, there are virtually no nullichiasmatic meiosis, as chiasmata play a crucial mechanistic role in linking homologous chromosomes during meiosis I. This observation is sometimes referred to as the “obliged crossing-‐over” rule. Secondly, multiple CO tend to occur farther apart than expected by chance alone. The latter phenomenon is called positive interference, i.e. the fact that a first CO diminishes the probability of a second one in its immediate vicinity. The degree of interference between intervals (say A-‐B vs B-‐C) can be measured on a 0 to 1 scale as: I = 1− DCOOBS DCOEXP where DCOOBS/DCOEXP is the ratio between the observed and expected numbers of double-‐ crossovers, also known as coefficient of coincidence. In the previous example, the coefficient of coincidence was ((4+6)/1000)/0.1*0.2 = 0.5, corresponding to an interference of 0.5. Mapping functions have been modified in order to account for the strong interference that is observed for closely linked intervals in many organisms, including mammals. The most commonly used mapping function is Kosambi’s one. It assumes that the level of interference diminishes with increasing distance. Centimorgans, as defined above, are a convenient additive measure of genetic distance. But what is the relationship between centimorgans and the physical measure of genetic distance, i.e. the base pairs? At small scale the relationship looks like a staircase: regions with very little recombination separated by “recombination hotspots”. The majority of CO indeed occur within ∼25,000 1-‐2Kb-‐ long recombination hotspots containing short recombination-‐triggering motifs. In human, the consensus sequence of the most common recombination-‐triggering motif is: CCNCCNTNNCCNC. At larger scale, one observes an increase in the recombination towards the telomeres where recombination hotspots tend to concentrate, and a comparative decrease in recombination around the centromeres, which are recombination cold-‐spots. Recombination varies between individuals, with sex having a major effect: the recombination rate is typically higher in the homogametic than in the heterogametic sex. But even within sex, recombination is variable and this is in part genetically determined. Of note, in women global recombination rate (i.e. the average number of CO in a gamete) is positively correlated with reproductive success. It has also been hypothesized that domestication has lead to an increase in recombination. Linkage plays a central role in genetics. As soon as recombinant DNA technology permitted the development of numerous polymorphic DNA markers (f.i. microsatellites and SNPs) for human and other species of interest, dense linkage maps comprising thousands of markers were generated. These played an essential role as a first step towards assembling the complete genomic sequence of these species, and – most importantly -‐ they permitted the mapping of thousands of genes underlying inherited defects and other monogenic traits, as well hundreds of Quantitative Trait Loci influencing complex diseases and quantitative traits in human, animals and plants (cfr. Chapter ...). Chromosomal sex determination In many species, the karyotype differs between the sexes. In placental mammals for instance, while females have n pairs of matched chromosomes, males have n-‐1 pairs of matched chromosomes (referred to as autosomes), plus one pair composed of two distinct chromosomes. This unique chromosomal pair corresponds to the sex chromosomes or gonosomes. In mammals, females are the homogametic sex and their n-‐th matched pair of chromosomes corresponds to two identical gonosomes referred to as “X”. The males are the heterogametic sex and their n-‐th unmatched gonosomal pair corresponds to one “X” (identical to the sex chromosomes of the females) and “Y” chromosome. The Y chromosome is typically considerably smaller than its X counterpart. As females are XX, all oocytes carry a X chromosome. On the other hand, halve the sperm cells carry a X and will give a female offspring on fertilization, while the other halve carry a Y and will give male offspring on fertilization. 120 million years ago, i.e. after the separation of the lineage that would give the metatheria (i.e. marsupials) and eutheria (i.e. placental mammals) from that of the prototheria (i.e. monotremes), a mutation in the SOX3 gene (encoding a transcription factor) located on a then regular autosome, generated an allele conferring maleness, known today as the SRY gene. The corresponding chromosomes carrying the original SOX3 allele Chapter 2: Genes in pedigrees Page 6/8 would become the X chromosome, while those carrying the newly born male-‐determining SRY gene would become the Y chromosome. How the size of the Y chromosome has been reduced during 120 million years of evolution, is a very interesting biological question. It is believed that mutations in genes in the vicinity of SRY created alleles that were beneficial for male fertility but deleterious for female fertility. Such new alleles would fare best in the population (and hence favored by natural selection) if they were confined to males. One way to achieve this would be to block recombination between such male-‐ beneficial haplotypes on the proto-‐Y chromosome and their X counterpart. As we will see later in this chapter, chromosomal inversions are one such recombination-‐blocking mechanism. It is now assumed that successive inversions have isolated increasing portions of the Y chromosome from its former X partner, reducing the portion along which the ancestral partners are still able to recombine (which is essential for proper segregation of the sex chromosomes during male meiosis) to the nowadays very short “pseudo-‐autosomal region” (PAR). The progressive degradation of the Y-‐specific region of the Y chromosome is thought to be a direct consequence of the fact that, contrary to the X chromosome, the Y never productively engages in recombination with a homologous partner (as YY individuals don’t occur) which seem essential for repair, and probably also of the fact that deleterious mutations on the Y are systematically shielded from purifying selection by the X chromosome. As the Y chromosome progressively lost an increasing proportion of its original gene complement, the ratio between autosomal gene copies (diploid) and gonosomal gene copies (diploid in females, haploid in males) started to differ between males and females, causing a problem for molecular systems requiring finely tuned component dosage. Countering this dosage issue is thought to underlie the selective pressure that let to the evolution of “dosage compensation” mechanisms to achieve proper and equal autosomal to gonosomal balance in both sexes. In mammals, dosage compensation involves two components. The first is “X-‐inactivation” in females (or “Lyonisation” in honor of Mary Lyon who discovered this mechanism). Although female cells are diploid XX, for most genes on the X-‐specific portion of the X chromosome, only one copy is active the other one being epigenetically silenced. In eutherian females, cells of the epiblast chose one of the X chromosomes to remain active, while the other one is silenced. This choice is to a large extent random, so that some cells will silence the paternal X while neighboring cells may have selected the maternal X for silencing. However, once the decision is made in an embryonic precursor cells, her choice is epigenetically transmitted to the entire clone of descending cells. All eutherian females are thus in essence “mosaics” (i.e. organisms comprising cells that derive from the same zygote yet differing in their genetic complement), and functionally haploid for many of the genes on the X chromosome. This functional mosaicism of females is directly apparent in tortoiseshell cats. These always female cats are heterozygous “B(lack)/O(range)” for a pigmentation gene on the X. The black sectors correspond to cell populations having inactivated the X carrying the O allele, while the orange sectors correspond to cell populations having inactivated the X carrying the B allele. The additional white spots, characterizing so-‐called calico cats, are due to the “Piebald” allele at a distinct autosomal locus. One of the key players of the X-‐inactivation process is the XIST gene, which is the only gene that is expressed from the inactive X and not from the active X. The corresponding XIST transcript is one of the best studied long non-‐coding RNA genes. It covers the X chromosome from which it is derived, thereby silencing it by a still rather obscure mechanism. In addition to its XIST coat, the inactive X chromosome is characterized by epigenetically inherited DNA methylation and chromatin silencing marks. The inactive X chromosome adopts a condensed heterochromatic conformation that is microscopically visible at the nuclear periphery as a so-‐called Barr body. X inactivation equalizes expression levels from the X chromosome in males and females. Proper gonosome-‐to-‐autosome balance is achieved by the second mechanism involved in dosage compensation, i.e. the doubling of expression levels of all genes on the X when compared to genes on autosomes. The mechanisms underlying this generalized X-‐specific boost of expression remains unknown. How does the SRY gene cause masculinization of the developing fetus? In a male fetus, the SRY gene is activated in somatic cells of the developing gonad, which consequently differentiate in Sertoli cells. Sertoli cells secrete signals which (i) cause the incoming germ cells to differentiate along the sperm rather than egg pathway, (ii) cause neighboring cells to differentiate into Leydig cells which secrete the sex-‐hormone testosterone which will cause masculinization of several organs, and (iii) block the development of the Müllerian anlagen (anti-‐Müllerian hormone). In the absence of SRY (female fetus), the somatic cells in the developing gonad differentiate in follicle cells, (i) letting incoming germ cells develop along the egg pathway, and (ii) stimulating neighboring cells to differentiate into estrogen-‐secreting theca cells. Chapter 2: Genes in pedigrees Page 7/8 In mammals, the male sex is heterogametic and the female sex homogametic. In some organisms, however, the situation is opposite: males are homogametic and females heterogametic. The gonosomes are then referred to as Z and W, males being ZZ and females ZW. This situation applies in particular to birds. As in the XY system, the W has a tendency to degenerate over time for what are assumed to be similar reasons. In these organisms, the sex-‐determining gene is not SRY. (Cell-‐autonomous sex differentiation in birds) Chapter 2: Genes in pedigrees Page 8/8