* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Deconstructed HS-PS1-2
Lewis acid catalysis wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Atomic orbital wikipedia , lookup
Organic chemistry wikipedia , lookup
Molecular orbital diagram wikipedia , lookup
Chemical warfare wikipedia , lookup
Computational chemistry wikipedia , lookup
Destruction of Syria's chemical weapons wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Resonance (chemistry) wikipedia , lookup
Livermorium wikipedia , lookup
Fine chemical wikipedia , lookup
California Green Chemistry Initiative wikipedia , lookup
Metallic bonding wikipedia , lookup
Chemical element wikipedia , lookup
Physical organic chemistry wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Transition state theory wikipedia , lookup
Drug discovery wikipedia , lookup
Bond valence method wikipedia , lookup
Electrochemistry wikipedia , lookup
Safety data sheet wikipedia , lookup
Al-Shifa pharmaceutical factory wikipedia , lookup
Stoichiometry wikipedia , lookup
Chemical reaction wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Chemical weapon proliferation wikipedia , lookup
Chemical weapon wikipedia , lookup
Chemical plant wikipedia , lookup
Chemical Corps wikipedia , lookup
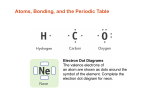
Periodic table wikipedia , lookup
Chemical potential wikipedia , lookup
Chemical industry wikipedia , lookup
Chemical bond wikipedia , lookup
History of molecular theory wikipedia , lookup
Electronegativity wikipedia , lookup
Electron configuration wikipedia , lookup
Atomic theory wikipedia , lookup
History of chemistry wikipedia , lookup
Extended periodic table wikipedia , lookup
VX (nerve agent) wikipedia , lookup
Moving NGSS to Instruction What is the intent of the performance expectation/learning? What are the key concepts for learning? What will students need to know or do to show mastery? HS-PS1-2 Construct and revise an explanation for the outcome of a simple chemical reaction based on the outermost electron states of atoms, trends in the periodic table, and knowledge of the patterns of chemical properties.[Clarification Statement: Examples of chemical reactions could include the reaction of sodium and chlorine, of carbon and oxygen, or of carbon and hydrogen.] [Assessment Boundary: Assessment is limited to chemical reactions involving main group elements and combustion reactions.] Knowledge Know and apply rules governing chemical reactions. Reasoning AND Skill/Performance* Distinguish between how element properties progress among families (vertical/patterns) compared to across families (horizontal/trend) on the periodic table. Determine an element’s valence state by identifying the number of electrons in the outer shell rather than number of electrons needed to fill the shell. Understand types of chemical bond and how they form. Explain how valence states of atoms determined. Identify rules for writing empirical chemical formulas. Construct a balanced chemical equation, meaning an equation with not net charge. Recognize that balanced chemical equations represent conservation of matter. Apply knowledge of valence electrons to develop balanced chemical equations. Know the structure of the periodic table. Recognize basic atomic structure. Draw conclusions/determine the chemical properties of an element (reactivity, electronegativity, valence, etc.) based on its position on the periodic table. Know that electrons are organized in orbital shells in accordance with a known set of rules. Understand that electronegativity describes the tendency of an element to attract electrons to it. *Refers to type of target Products