* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download ENERGETICS
Radical (chemistry) wikipedia , lookup
Phosphorylation wikipedia , lookup
Basal metabolic rate wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Metalloprotein wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Photosynthesis wikipedia , lookup
Electron transport chain wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Microbial metabolism wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Citric acid cycle wikipedia , lookup
Biochemistry wikipedia , lookup
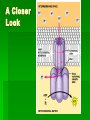
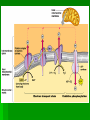
ENERGETICS Laws of Thermodynamics #1 – Energy can be transferred and transformed, but not created or destroyed. (Conservation of energy) #2 – Every energy transfer /transformation increases the entropy (disorder) of the universe. Coupled Reactions Living organisms appear to “cheat” the thermodynamic laws because they develop “order”, however, they are essentially “open systems” and receive and release energy by coupling reactions of metabolism. Metabolism All the chemical processes in the body can be categorized as either anabolic (energy-requiring in order to build up) or catabolic (energy-releasing for breakdown). They work in concert with each other to meet the needs of the organism. Respiration The process of extracting stored energy from glucose to form ATP C6H12O6 + 6O2 6CO2 + 6H20 + ATP(energy) Aerobic Respiration Consists of 3 phases Glycolysis Krebs Cycle Oxidative Phosphorylation aka Electron Transport Chain Glycolysis Literally means “sugar-breaking” Glucose is broken down in a series of reactions, each by an enzyme. Magnesium ions (Mg2+) are cofactors that aid enzyme action. Takes place in the cytoplasm. The final product from the breakdown of glucose is pyruvic acid. In order to start the reactions some activation energy is supplied in the form of 2 ATP molecules. Glycolysis Summary 2 ATP supply energy. 2 NADH (coenzyme) are produced as electrons are released during the breakdown. 4 ATP are produced. 2 pyruvic acid molecules (3 C) result. Net gain of 2 ATP. Crossroads Depending on the presence of oxygen, determines what happens to the pyruvic acid next. Without oxygen, the process of fermentation will occur. We will explore that process later. But we can extract more energy, if O2 is present, let’s take a look! Pyruvic acid (pyruvate) binds to coenzyme A to form acetyl CoA, releasing CO2 and electrons (picked up by NADH) just prior to entering the cycle. (2 C) Kreb’s Cycle This cycle of reaction occurs in the matrix of the mitochondrion. Although the Kreb’s cycle does not use oxygen directly, the molecules required to keep it running, do require it in order to be recycled. Kreb’s Cycle In the course of the cycle, the initial molecule (Acetyl CoA) is, first combined with a 4C compound (Oxaloacetate) to form a 6C compound (Citric Acid*) and then subsequently broken apart, piece by piece w/ more energy and CO2 released. *Kreb’s cycle aka Citric Acid Cycle. Watch the cycle in motion. Please note that this animation is oversimplified, but you should see the basic breakdown of the molecule and the subsequent results. Kreb’s Cycle* Intermediate Reactions The breakdown reactions can generally be categorized as one of the three: Phosphorylation Redox Isomerization *also Glycolysis intermediate reactions A Closer Look Phosphorylation – changes the shape of the molecule, thus allowing work to be performed. A Closer Look Redox reactions release energy when electrons move closer to electronegative atoms. The loss of electrons is called oxidation. The addition of electrons is called reduction. More generally: Xe- + Y -> X + YeX, the electron donor, is the reducing agent and reduces Y. Y, the electron recipient, is the oxidizing agent and oxidizes X. If the degree of electron sharing changes it is also considered a redox reaction. A Closer Look An example of redox is when hydrogen atoms are stripped from glucose and passed to a coenzyme, like NAD+ (nicotinamide adenine dinucleotide). Enzymes strip two hydrogen atoms from glucose, pass two electrons and one proton to NAD+ and release H+. This changes the oxidized form, NAD+, to the reduced form NADH. A Closer Look Isomerization – molecule changes shape but retains the same molecular formula. Kreb’s Cycle Summary The released energy is picked up by 1 ATP, 3 NADH and 1 FADH2. 1 molecule of CO2 is released. The CO2 is considered waste and we exhale it. Since two molecules of pyruvate, from the 1 glucose molecule, go through this cycle, the amounts above are actually doubled. A Closer Look Summary so far… Glucose Molecule 2 pyruvate 6 Carbon dioxide ATP NADH Net gain of 4: 10 total: 4-2=2 8 Kreb’s glycolysis 2 Kreb’s 2 glycolysis FADH2 2 Kreb’s Electron Transport Chain aka Electron Transport System Occurs in the cristae of mitochondrion. Electrons from NADH & FADH2 are passed (like a hot potato) through a chain of cytochrome molecules. This regenerates NAD+ and FAD so that they can be reused in glycolysis and Kreb’s cycle. ETC or ETS Oxygen is needed to accept the electrons, together with H+ ions, at the end of the chain, forming water. More importantly, a lot of ATP is generated through this process called oxidative phosphorylation. A Closer Look •Electrons carried by NADH are transferred to the first molecule in the electron transport chain, flavoprotein. •The electrons carried by FADH2 have lower free energy and are added to a later point in the chain. Oxidative Phosphorylation The maximum output from ox-phos is 3 ATP/NADH and 2 ATP/FADH2 Calculate how many ATP can be produced during this process from the 1 molecule of glucose. 34 Chemiosmosis A major part of oxidative phosphorylation, this basic process also occurs in chloroplasts. The energy lost from electrons passing through the ETS, is used to phosphorylate ADP to ATP. (coupling reactions) Chemiosmosis involves coupled reactions, where the products of one reaction are used in another reaction. In this case, the initial products are H+ ions, which are released from NADH and FADH2. These protons are pumped out of the fluid matrix, across the cristae, to the intermembrane space of the mitochondrion. A pH and electrical gradient is formed as the protons accumulate, forming a reservoir of potential energy. The protons flow back into the matrix through channel proteins called ATP synthases. This flow generates the energy to produce ATP. At the end of the ETS, the moving electrons, which first served to provide the H+ ions (protons) when the bonds of NADH and FADH2 were broken, are transferred to oxygen and coupled with the pumped H+ ions (back in the matrix), form water. A Closer Look http://www.youtube.com/watch?v=3y1dO4nNaKY How efficient is respiration in generating ATP? Complete oxidation of glucose releases 686 kcal per mole. Formation of each ATP requires at least 7.3 kcal/mole. Efficiency of respiration is 7.3 kcal/mole x 38 ATP/glucose/686 kcal/mole glucose = 40%. The other approximately 60% is lost as heat. Cellular respiration is remarkably efficient in energy conversion. Whew! Now you can see why cell respiration leads to some of the top 40 ways you know you’ve been traumatized by AP Biology. In the end, if you remember nothing else, remember what results after each phase or cycle. It’s not quite over yet, remember we said that there is an alternative path if no oxygen was present? Well……. FERMENTATION How Glycolysis keeps going if there is no oxygen. When there is no Oxygen why is Fermentation necessary? W/O O2, glycolysis is the ONLY chemical reaction to release energy from glucose. Cells need to continuously carry out glycolysis, but eventually the NAD can be used up. If all the NAD is used up, then glycolysis jams or stops. Why would it be disastrous for the cell if glycolysis stops? It would run out of energy. What does Fermentation do in order to help glycolysis continue? In Fermentation, chemical reactions occur that free up the NAD, thus regenerating it for use in glycolysis. The electron energy released from NADH+ (What NAD is called when it carries the high energy electrons) is put back into pyruvic acid. This chemical reaction results in a new product. Fermentation ONLY occurs if there is no oxygen. Products of Fermentation In animals, the new product formed is lactic acid. This often forms in muscles when they can’t get the oxygen they need fast enough. Lactic acid accumulating in muscles is painful, but this chemical can later be broken down to extract energy when enough oxygen is present. Products of Fermentation In yeast, the new products formed are alcohol and carbon dioxide. People put both these products to use to make wines and cause bread dough to rise. Fermentation Review In your notes write your answers in your own words. How does fermentation help a cell release energy from glucose? Compare and contrast lactic acid fermentation and alcoholic fermentation. What would happen to fermenting wine if there was an air leak in the fermentation tank?