* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Telomere Shortening and Tumor Formation by Mouse Cells Lacking
History of RNA biology wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Point mutation wikipedia , lookup
Microevolution wikipedia , lookup
Genealogical DNA test wikipedia , lookup
Epigenetics in learning and memory wikipedia , lookup
X-inactivation wikipedia , lookup
Gene therapy of the human retina wikipedia , lookup
Cre-Lox recombination wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Epigenetics in stem-cell differentiation wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
Genome editing wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
Designer baby wikipedia , lookup
Primary transcript wikipedia , lookup
No-SCAR (Scarless Cas9 Assisted Recombineering) Genome Editing wikipedia , lookup
Mir-92 microRNA precursor family wikipedia , lookup
History of genetic engineering wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Non-coding RNA wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
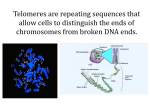
Telomere Shortening and Tumor Formation by Mouse Cells Lacking Telomerase RNA Maria A. Blasco et al. (1997), Cell Vol. 91, p. 25 - 34 Outline 1. Introduction 2. Objective 3. Results 4. Summary 5. Take-Home-Lesson 6. Discussion 2 Introduction Telomeres: -at the end of chromosomes -repetitive DNA (vertebrates: (TTAGGG)n ) -contain non-coding DNA material -protect chromosomes -prevent fusions and degradation and a constant loss of important DNA from chromosome ends http://www.wissenschaft-online.de/sixcms/ media.php/912/thumbnails/Telomer1.jpg.644586.jpg 3 Introduction Problem: the chromosomes shorten with every cell divison Reason: DNA-Polymerase moves in the 5‘ to 3‘ direction and needs RNAPrimer to start the building of okazaki fragments (for lagging strand). Replication is possible as long as RNA-Primer can bind to the http://upload.wikimedia.org/wikipedia/commons/9/93/Dn areplication.png template stand 4 Introduction Solution: enzyme telomerase -ribonucleoprotein with reverse transcriptase activity → RNA – component serves as a template for the telomere repeats (template region of telomerase is 3'-CAAUCCCAAUC-5’) → adds DNA sequence repeats (5’-TTAGGG-3’ in all vertebrates) to the 3’ end of DNA strands in the telomere region 5 Introduction Telomerase binds to 3‘ end of the leading strand, that is complementary to the telomerase RNA RNA-component is used as template → adding of repeating sequence (5‘-TTAGGG-3‘) is possible the DNA-Polymerase complements the lagging strand http://en.wikipedia.org/wiki/File: Working_principle_of_telomerase.png 6 Objective The objective is to describe the effects of the absence of telomerase activity on telomere length, cellular viability, neoplastic transformation and tumor formation in nude mice. 7 Results Goal 1: Generation of a knockout mouse The telomerase RNA component (mTR) is necessary for telomerase activity → RNA component functions as template To generate a mouse deficient for telomerase activity, the mTR gene was deleted from the mouse germline Therefore they constructed the plasmid pPNT-mTRΔ, which allows replacement of the mTR gene with the neomycin resistance gene (homologous recombination) 8 Results 9 Results Positive and negative selection of ES cells When homologous recombination was successful, the ES cells are resistant against ● Neomycin → antibiotic resistance ● Ganciclovir → loss of the marker HSV thymidine kinase (HSV-TK) 10 Results 11 Results Southern Blot: 1: mTR+/+ wildtype 2: mTR+/- heterozygous 3: mTR-/- homozygous 12 Results Observation: The generated mTR-/- mice were able to live → mTR is not essential for embryonic development 13 Results Goal 2: Determination of fertility Crossing first generation G1 mTR-/- mice to each other → G2 mTR-/- mice were obtained → mTR is not essential for fertility For the 6 generations, which were analyzed, all mice were alive. To get a new generation, they always crossed two mice from the previous generation to each other 14 Results Goal 3: Detection of telomerase activity in mTR-/- mice and mouse embryonic fibroblasts (MEF) MEF cultures and S100 extracts from brain, liver, thymus and spleen were tested for telomerase activity with the TRAP assay TRAP assay is based on the PCR amplification of a substrate, which is extended by telomerase 15 Results PCR for the MEF cultures 16 Results Observation: Telomerase activity was detected in mTR+/+ and mTR+/MEF cultures and S100 extracts → TRAP assays showed the absence of telomerase activity in mTR-/- embryonic fibroblasts and adult mice tissues → mTR gene is essential for mouse telomerase activity in vivo 17 Results Goal 4: Recovering of telomerase activity of mTR-/- MEFs mTR gene was reintroduced into mTR-/MEF cultures by transient transfection 18 Results Observation: 48 h after the transfection the MEF cultures had telomerase activity The cells, transfected with an empty plasmid, had no telomerase activity → telomerase RNA is responsible for the telomerase activity. → mTR-/- MEFs are able to express telomerase activity after reintroduction of the mTR gene into the cells 19 Results Goal 5: Examination of the growth of mTR-/- MEF cultures MEF cultures were generated from mTR+/+ , mTR+/- and mTR-/- G1 embryos and from mTR-/- G2, G3, G4 and G6 embryos 20 Results Observation: ● the growth rates of all MEFs were similar → missing telomerase activity does not inhibit the cell division 21 Results Goal 6: Determination of telomere length in mTR-/- cells MEF cultures were derived from G1 wildtype embryos and from mTR-/- G1 - G6 embryos Southern Blot: Genomic DNA was blotted and probed with a TTAGGG-probe to detect terminal fragments → it is not obvious, that telomeres of mTR-/- generations are shorter than the ones of the wildtype 22 Results Same treatment with MEF cultures from G1 wildtype and from mTR-/- G1 – G4 animals but this time DNA was isolated from cells during early, mid and late passage → telomere fragments shorten with increasing cell doublings in culture, when telomerase is absent 23 Results Fluorescence in situ hybridization (FISH) MEF cultures from G1 WT and mTR-/- G2, G4 and G6 animals → all chromosome ends were examined with a (TTAGGG)probe, which had a fluorescence-tag Definition: 1 telomere fluorescence unit (TFU) represents 1 kb of (TTAGGG)-repeats 24 Results Observation: The TTAGGG-signal decreases with increasing mTR-/generations 25 Results FISH images: In G6 cultures there was no TTAGGG-signal for 5% of the chromosomes end-to-end-associations of chromosomes were observed more frequently → no telomerase activity leads to instability 26 Results Goal 7: Examination of tumor formation in the absence of telomerase activity MEF cultures from - G1 mTR+/+ - G1 mTR+/- G1, G2, G3, G4 and G6 mTR-/- mice Infection with retrovirus, carrying the genes for adenovirus E1A, activated protooncogene rasv12 and puromycin drug resistance → oncogenic transformation → foci were selected and 105 cells were injected in nude mice 27 Results Tumors were generated from the mTR+/+ , mTR+/- and all of the mTR-/- cells → telomerase activity is not essential for transformed cells to form tumors in nude mice 28 Summary • the telomerase RNA component (mTR) is required for telomerase activity • telomerase activity is not necessary for embryonic development or fertility • the absence of telomerase has no influence on the growth rates of cultures • Without telomerase, the telomere length decreases from generation to generation and also during cell doublings in culture • telomerase is not responsible for tumor formations 29 Take-Home-Lesson Telomerase is necessary for telomere length conservation but is not essential for establishment of cell lines, oncogenic transformation or tumor formation. 30 Discussion 1. Wie werden Knockout-Mäuse erzeugt? Beschreiben sie stichwortartig das Vorgehen. 2. Welche Aufgabe hat die Telomerase? 31 Welche Aufgabe hat die Telomerase? Die Telomerase ist ein Ribonukleoprotein und wirkt als reverse Transkriptase, wobei die RNA-Komponente als Matrize bei der Verlängerung der Telomere dient. →Telomerase verlängert Telomere und wirkt somit einer Verkürzung der Chromosomen bei der Replikation entgegen →Telomerase verhindert den Verlust essentieller genetischer Information nach jeder Replikationsrunde 32