* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Non-competitive
Adenosine triphosphate wikipedia , lookup
Point mutation wikipedia , lookup
Gaseous signaling molecules wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Lipid signaling wikipedia , lookup
Metabolic network modelling wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Ultrasensitivity wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Restriction enzyme wikipedia , lookup
Western blot wikipedia , lookup
Lactate dehydrogenase wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Citric acid cycle wikipedia , lookup
Catalytic triad wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Metalloprotein wikipedia , lookup
Proteolysis wikipedia , lookup
Biochemistry wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Biosynthesis wikipedia , lookup
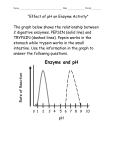
Apoenzyme – the polypeptide portion of an enzyme Cofactor – non protein portion of an enzyme May be a metal ion such as Zn2+ of Mg2+ May also be an organic molecule such as vitamin B or heme – called a coenzyme Substrate – the molecule an enzyme acts on Activation – any process that initiates or increases the action of an enzyme Inhibition – any process that inactivates an enzyme or reduces its effectiveness Competitive inhibitor – binds at the active site Non-competitive inhibitor – binds at other than the active site Enzyme Catalysis • Enzyme: a biological catalyst – with the exception of some RNAs that catalyze their own splicing, all enzymes are proteins – enzymes can increase the rate of a reaction by a factor of up to 1020 over an uncatalyzed reaction – some catalyze the reaction of only one compound ( NH2 )C=O + H2 O urease Urea 2NH3 + CO2 – others are stereospecific; for example, enzymes that catalyze the reactions of only L-amino acids – others catalyze reactions of specific types of compounds or bonds; for example, trypsin that catalyzes hydrolysis of peptide bonds formed by the carboxyl groups of Lys and Arg Mechanism of Action – both the lock-and-key model and the induced-fit model emphasize the shape of the active site – however, the chemistry of the active site is the most important – just five amino acids participate in the active sites in more than 65% of the enzymes studies to date – these five are His > Cys > Asp > Arg > Glu – four these amino acids have either acidic or basic side chains; the fifth has a Enzyme Regulation • Protein modification: the process of affecting enzyme activity by covalently modifying it - • the best known examples of protein modification involve phosphorylation/dephosphorylation example: pyruvate kinase (PK) is the active form of the enzyme; it is inactivated by phosphorylation to pyruvate kinase phosphate (PKP) ATP ADP active inactive kinase PK PKP phosphatase Pi Succinylcholine is a competitive inhibitor of acetylcholine at receptors – it is broken down slowly by acetylcholinesterase. This leaves the receptor blocked and the muscle relaxed. Chem Connect 22A, p.555 Sulfa drugs are competitive inhibitors of p-aminobenzoic acid in the synthesis of folic acid in bacteria Chem Connect 22D, p.562 Enzyme Regulation • Proenzyme (zymogen): an inactive form of an enzyme that must have part of its polypeptide chain cleaved before it becomes active. An example is trypsin, a digestive enzyme - it is synthesized and stored as trypsinogen, which has no enzyme activity. It becomes active only after a six-amino acid fragment is hydrolyzed from the N-terminal end of its chain Removal of this small fragment changes in not only the primary structure but also the tertiary structure, allowing the Enzyme Regulation • Isoenzyme: an enzyme that occurs in multiple forms; each catalyzes the same reaction Example: lactate dehydrogenase (LDH) catalyzes the oxidation of lactate to pyruvate The enzyme is a tetramer of H and M chains. H4 is present predominately in heart muscle. M4 is present predominantly in the liver and in skeletal muscle. H3M, H2M2, and HM3 also exist – H4 is allosterically inhibited by high levels of pyruvate while M4 is not. H4 in serum correlates with the severity of heart