* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download PowerPoint Presentation - No Slide Title
Aromaticity wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Stille reaction wikipedia , lookup
Ene reaction wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
Petasis reaction wikipedia , lookup
Asymmetric induction wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Marcus theory wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Hydroformylation wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
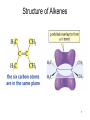
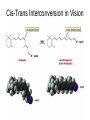
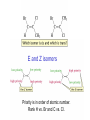
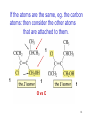
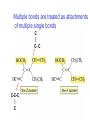
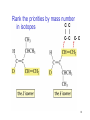
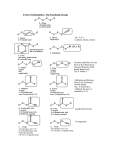
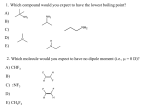
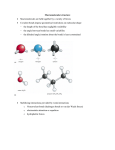
Chapter 3 Alkenes: Structures, Nomenclature and an Introduction to Reactivity Thermodynamics and Kinetics Adapted from Profs. Turro & Breslow, Columbia University and Prof. Irene Lee, Case Western Reserve University 1 Ever put an apple into a bag with green tomatoes or a green banana? Natural Products: CH2=CH2 What might account for the difference between lemon and orange in the limonene structure? 2 Molecular Formulas of Alkenes Saturated vs. Unsaturated: Missing Hydrogens Alkanes are completely “saturated” i.e. only single bonds Each double bond has 1 degree of unsaturation. Each ring has 1 degree of unsaturation. Each triple bond is 2 degrees of unsaturation. Compare a molecular formula to an alkane’s: every TWO Hydrogens less = I degree of unsaturation 3 Molecular Formulas of Alkenes Saturated vs. Unsaturated: Missing Hydrogens Noncyclic alkene: CnH2n 1 degree of unsaturation Cyclic alkene: CnH2n-2 (Same as an alkyne; 2 degrees of unsaturation) 4 Systematic Nomenclature of Alkenes •Follows alkane rules; treats double bond as a function: Think of alcohols 5 •Substituents in alphabetical order with lowest numbers 6 •Cyclic alkenes: 7 Important Special Terms Vinyl Hs: bonded to the double bond. Allylic Hs: on sp3 carbons next to the double bond. 8 Structure of Alkenes 9 Isomers of Alkene 10 Dipole Moments of Alkene Isomers 11 Cis-Trans Interconversion in Vision 12 QuickTime™ and a Video decompressor are needed to see this picture. 13 E and Z isomers Priority is in order of atomic number. Rank H vs. Br and C vs. Cl. 14 Naming using E, Z E (entgegen:opposite): Z (zusammen; same) Consider the atomic number of the atoms bonded directly to a specific sp2 carbon 1,1 on same side = Z 1 2 1 2 1,1 on opposite sides = E 1 1 15 If the atoms are the same, eg. the carbon atoms: then consider the other atoms that are attached to them. 1 1 1 1 O vs C 16 Multiple bonds are treated as attachments of multiple single bonds C | C- C C-C-C | C 17 Rank the priorities by mass number C C in isotopes | | C- C C- C 18 An alkene is an electron rich molecule, a nucleophile. “nucleophile”- likes nuclei (likes protons: H+) Nucleophiles: electron-rich atoms or molecules that react with electrophiles. “electrophile”- likes electrons (likes minus: eand anions) Examples of nucleophiles 19 Nucleophiles are attracted to electron-deficient atoms or molecules (electrophiles) Examples of Electrophiles 20 Electrophilic Addition of HBr to Alkene A two step reaction. Mechanistic path of a reaction: how reactants form products. How can a mechanism be illustrated? i.e. bond making & bond breaking 21 Using Curved Arrows in Reaction Mechanisms Movement of a pair of electrons: START arrows from electrons pointing to electrophile Use 1/2 arrow for the movement of one electron 22 Using Curved Arrows 23 A Reaction Coordinate Diagram Transition states have partially formed bonds Intermediates have fully formed bonds 24 Thermodynamic Parameters DGo = DHo - TDSo Gibbs standard free energy change (DGo) Enthalphy (DHo): the heat given off or absorbed during a reaction Entropy (DSo): a measure of freedom of motion If DSo is small compared to DHo, DGo ~ DHo 25 Exergonic Reaction -DGo Endergonic Reaction +DGo 26 DH o for any reaction can be calculated from bond dissociation energies D D D D D D D D 27 Kinetics deals with the rate of chemical reactions and the reaction mechanism Rate of a reaction = number of collision per unit time fraction with fraction with X sufficient energy X proper orientation The rate-limiting step controls the overall rates of the reaction 28 The free energy of activation & the transition state and the reactants 29 DG‡ = DH‡ -TDS‡ DG‡ : (free energy of transition state)- (free energy of reactants) DH‡ : (enthalpy of transition state) - (enthalpy of reactants) DS‡ : (entropy of transition state) - (entropy of reactants) 30 Rates and Rate Constants First-order reaction Second-order reaction A A+B rate = k [A] B C+D rate = k [A][B] 31 The Arrhenius Equation -Ea/RT Ea = DH‡ +RT k = Ae Rate Constants and the Equilibrium Constant A k1 B k-1 Keq = k1/k-1 = [B]/[A] 32 Transition State Versus Intermediate intermediate intermediate Transition states have partially formed bonds Intermediates have fully formed bonds 33 Electrophilic Addition of HBr to 2-Butene DG0 ‡ The rate-limiting step controls the overall rates of the 34 Reaction. It has the highest activation energy. QuickTime™ and a Cinepak decompressor are needed to see this picture. 35 36