* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Relationships between dinoflagellate cyst assemblages in surface
History of research ships wikipedia , lookup
Pacific Ocean wikipedia , lookup
Atlantic Ocean wikipedia , lookup
Physical oceanography wikipedia , lookup
Marine habitats wikipedia , lookup
Marine pollution wikipedia , lookup
Marine biology wikipedia , lookup
The Marine Mammal Center wikipedia , lookup
Sea in culture wikipedia , lookup
Effects of global warming on oceans wikipedia , lookup
Geology of the North Sea wikipedia , lookup
Arctic Ocean wikipedia , lookup
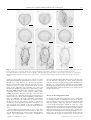
JOURNAL OF QUATERNARY SCIENCE (2001) 16(7) 667–680 Copyright 2001 John Wiley & Sons, Ltd. DOI: 10.1002/jqs.652 Relationships between dinoflagellate cyst assemblages in surface sediment and hydrographic conditions in the Bering and Chukchi seas TAOUFIK RADI,* ANNE DE VERNAL and ODILE PEYRON Centre de recherche en géochimie isotopique et en géochronologie (GEOTOP), Université du Québec à Montréal, P.O. Box 8888, Montréal, QC H3C 3P8, Canada Radi, T., de Vernal, A. and Peyron, O. 2001. Relationships between dinoflagellate cyst assemblages in surface sediment and hydrographic conditions in the Bering and Chukchi seas. J. Quaternary Sci., Vol. 16 pp. 667–680. ISSN 0267-8179. Received 3 April 2001; Revised 23 July 2001; Accepted 27 July 2001 ABSTRACT: Palynological analyses were performed on 52 surface sediment samples from the eastern part of the Bering Sea and Chukchi Sea in order to document the regional distribution of dinoflagellate cyst assemblages and their relationship with sea-surface conditions. The assemblages present a relatively high species diversity (20 taxa are recovered routinely), especially in the Bering Sea, where they are dominated by Operculodinium centrocarpum and the cyst of Pentapharsodinium dalei accompanied mainly by Spiniferites elongatus s.l., Spiniferites ramosus, Impagidinium pallidum, Brigantedinium spp., Islandinium minutum, Selenopemphix quanta, Selenopemphix Journal of Quaternary Science nephroides, Quinquecuspis concreta and the cyst of Polykrikos kofoidii. The percentages of the main taxa vary with latitude, and principal component analysis shows that the distribution of assemblages is closely related to hydrographic conditions, notably the seasonal duration of sea-ice cover and the sea-surface temperature in February. The dinoflagellate cyst assemblages from the Bering Sea differ significantly from those of subarctic seas of the North Atlantic, with respect to their species composition and relationships with sea-surface conditions. In particular, the occurrence of the cyst of Polykrikos kofoidii and Quinquecuspis concreta and the positive correlation between the percentages of Operculodinium centrocarpum and the extent of sea-ice, constitute peculiar features in the Bering Sea assemblages. Copyright 2001 John Wiley & Sons, Ltd. KEYWORDS: Bering Sea; Chukchi Sea; dinocysts; sea-ice cover; sea-surface temperature. Introduction Palynological studies of surface sediment samples from middle to high-latitude marine environments revealed that dinoflagellate cysts (dinocysts) are excellent tracers of hydrographic conditions, including the sea-surface temperature, salinity and seasonal duration of sea-ice cover (e.g. Mudie, 1992; Rochon and de Vernal, 1994; Matthiessen, 1995; Marret and de Vernal, 1997; de Vernal et al., 1997, 2000). These studies led us to hypothesise that dinocysts also could be good tracers of sea-surface conditions in subpolar regions of the North Pacific Ocean, such as the Bering Sea, and in adjacent Arctic seas, such as the Chukchi Sea. To date, however, only a few studies on high-latitude dinocyst assemblages from the North Pacific Ocean and western Arctic Ocean are available. They deal with * Correspondence to: T. Radi, GEOTOP, Université du Québec à Montréal, P.O. Box 8888, Montréal, Québec, H3C 3P8, Canada. E-mail: [email protected] Contract/grant sponsor: Fonds FCAR of Quebec. Contract/grant sponsor: Natural Sciences and Engineering Council of Canada. modern assemblages from marine environments around Japan (cf. Matsuoka, 1985, 1987, 1992), the Cenozoic biostratigraphy of the northwest North Pacific and Bering Sea (Bujak, 1984; Bujak and Matsuoka, 1986; Matsuoka and Bujak, 1988), and the Quaternary ecostratigraphy of the Gulf of Alaska (de Vernal and Pedersen, 1997; Marret et al., 2001). Here, we present the results of palynological analyses performed on surface sediment samples from 52 sites of the eastern Bering Sea and Chukchi Sea (Fig. 1 and Table 1). Our objective is to determine the relationships between the distribution of dinocyst assemblages and the hydrographical parameters of the upper water layer, notably the salinity and temperature and the seasonal duration of sea-ice cover. We also examined possible links between the dinocyst assemblages and the primary productivity. This study is a contribution to a collective project aiming at the establishment of a hemispheric data base (cf. de Vernal et al., this issue). Such a data base should permit wider domain application of the transfer functions developed initially for palaeoceanographic reconstruction in the northern North Atlantic Ocean and adjacent subpolar basins (e.g. de Vernal et al., 1994, 1997, 2000; Rochon et al., 1999). 668 JOURNAL OF QUATERNARY SCIENCE 70°N 160°E 140°E 180° −160°W −140°W −130°W −120°W 60°N −120°W 130°E 0m 10000m 2 Chukchi Sea 140°E −130°W Siberia 60°N Alaska m 20000m 10 150°E 50°N −140°W Bering Sea s nd 50°N n a Isl tia eu Al 160°E 170°E 180° −170°W −160°W −150°W Figure 1 Map showing locations of study sites. The bathymetry is represented by isobaths at 200 and 1000 m Oceanographic context Hydrography A latitudinal gradient of sea-surface temperature is observed in the study area, which includes the Bering Sea and Chukchi Sea (Table 2). From the north to the south, temperature varies between freezing point (−1.9 ° C) and 5 ° C in February, and between 7 and 12 ° C in August (cf. NODC, 1994). A gradient of summer salinity also is observed from the Chukchi Sea (26.7 to 28) to the southern Bering Sea (30 to 33). The seasonal duration of sea-ice cover (>50% of sea-ice coverage) ranges from zero to five months per year in the Bering Sea and from six to twelve months per year in the Chukchi Sea. The extension of sea-ice cover is controlled by atmospheric circulation, which initiates considerable interannual fluctuations of the surface coverage and thickness of sea-ice (Cavalieri and Parkinson, 1987; Niebauer, 1988; Cavalieri and Martin, 1994; Roach et al., 1995). In the northeast Bering Sea, three hydrographic domains can be distinguished depending upon freshwater inputs and surface currents (Cooney and Coyle, 1982; Walsh and McRoy, 1986; see Fig. 2). 1 The Anadyr Water (AW) to the west is characterised by low summer temperature (ca. 3 ° C) and salinity of about 33. It circulates northeastward through the Anadyr Strait and constitutes an important source of nutrients (20 to 40 µM of nitrates) owing to the upwelling from the Aleutian basin on to the Bering Sea shelf (Walsh et al., 1989; Springer and McRoy, 1993; Grebmeier and Cooper, 1995). The annual productivity of this water mass is about 300 gC m−2 (Grebmeier, 1993; Hansell et al., 1993). 2 The Alaska Coastal Water (ACW) is relatively warm (about 11 ° C) and characterised by low salinity (about 32) during summer because of freshwater inputs from the Yukon River, which also contributes to some nutrient enrichment (Walsh et al., 1989; Chen, 1993; Chen et al., 1997). However, the Copyright 2001 John Wiley & Sons, Ltd. nitrate content of the water, which circulates northwards along the Alaskan coast, considerably decreases (<1 µM) after the spring bloom. As a consequence, the annual productivity associated with this water mass is relatively low (<60 gC m−2 ; Grebmeier, 1993; Hansell et al., 1993). 3 The Bering Shelf Water (BSW) formed by waters from the North Pacific Current spreads on the shelf, which constitutes a more open ocean environment characterised by a salinity of approximately 32.5 in summer (Thomson, 1981; Reed and Stabeno, 1997). This water mass mixes with the AW in the north, where productivity is relatively low (Maynard and Clark, 1987; Hansell et al., 1993). In the Chukchi Sea, surface waters result from the mixing of Arctic waters with a subpolar component originating from the Bering Sea. As a consequence, in summer, the surface water is characterised by salinity in the range 26.5–27, and by a northward gradient of decreasing temperature from 7 ° C to freezing conditions. The northward circulation of the Bering Sea water (Fig. 2) through the Bering Strait (85 km wide and 50 m deep) is controlled by the sea-level difference of about 0.5 m between the Pacific and Arctic Oceans (Overland and Roach, 1987). The northward flow of surface water on the Chukchi shelf is further determined by atmospheric circulation patterns (Coachman, 1993). The mean annual water flux towards the Arctic through the Bering Strait is estimated to be about 0.83 Sv (Roach et al., 1995), but presents strong interannual and seasonal variations (Aagaard et al., 1985; Overland and Roach, 1987; Coachman and Aagaard, 1988; Roach et al., 1995). The circulation of waters rich in nutrients towards the Arctic Ocean, notably that coming from the Anadyr Gulf neighbourhood towards the Bering Strait, plays a role by bringing nutrients to the Arctic Ocean (Springer and McRoy, 1993). However, the concentration of nutrients strongly decreases as the current circulates northward (Walsh et al., 1989; Hansell et al., 1993; Cooper et al., 1997) and the resulting productivity is low (Maynard and Clark, 1987; Hansell et al., 1993). J. Quaternary Sci., Vol. 16(7) 667–680 (2001) DINOCYSTS IN SURFACE SEDIMENT, BERING AND CHUKCHI SEAS Table 1 669 Sampling site number and location Site number Laboratory number Coring devise Latitude Longitude Water depth(m) 1565-5 1322-3 1322-2 1322-1 1565-6 1566-1 1566-2 1570-1 1565-4 1566-6 1570-6 1565-3 1565-2 1565-1 1570-2 1455-4 1461-5 1566-5 1461-4 1566-4 1461-6 1455-1 1461-1 1461-3 1461-2 1455-2 1566-3 1477-1 1477-2 1455-3 1477-3 1454-1 1477-4 1454-2 1465-1 1454-6 1465-2 1455-5 1454-3 1477-5 1477-6 1465-3 1530-4 1530-3 1454-5 1465-4 1454-4 1465-6 1530-5 1465-5 1455-6 1530-6 Gravity Box Box Box Gravity Gravity Gravity Gravity Gravity Gravity Gravity Gravity Gravity Gravity Gravity Gravity Gravity Gravity Gravity Gravity Gravity Gravity Gravity Gravity Gravity Gravity Gravity Gravity Gravity Gravity Gravity Gravity Gravity Gravity Gravity Gravity Gravity Gravity Gravity Gravity Gravity Gravity Gravity Gravity Gravity Gravity Gravity Gravity Gravity Gravity Gravity Gravity 76° 15.00N 75° 44.03N 74° 30.06N 74° 00.00N 71° 45.00N 67° 52.00N 63° 03.00N 63° 00.70N 63° 00.00N 62° 39.80N 62° 32.10N 63° 00.00N 62° 29.50N 62° 00.00N 61° 08.90N 61° 18.00N 61° 07.60N 61° 07.20N 60° 59.00N 60° 49.00N 60° 45.00N 60° 38.50N 60° 01.40N 60° 33.60N 60° 17.50N 59° 58.90N 59° 54.80N 59° 45.20N 59° 35.70N 59° 29.30N 59° 14.00N 58° 53.70N 58° 34.20N 58° 27.30N 58° 22.00N 58° 04.50N 57° 18.20N 57° 52.30N 57° 07.30N 57° 02.50N 56° 38.20N 56° 23.80N 56° 20.80N 56° 28.90N 57° 15.00N 56° 03.50N 55° 24.00N 55° 39.00N 55° 18.70N 55° 12.20N 54° 55.00N 53° 45.10N 167° 00.00W 160° 51.63W 159° 58.06W 161° 23.07W 156° 30.00W 167° 55.00W 174° 27.00W 173° 01.90W 175° 00.00W 175° 38.70W 174° 43.00W 177° 00.00W 177° 02.00W 178° 00.00W 172° 18.30W 176° 29.80W 175° 12.10W 173° 25.60W 176° 44.00W 177° 03.00W 174° 36.00W 175° 08.00W 170° 37.70W 176° 31.80W 175° 54.30W 171° 01.50W 176° 19.50W 174° 24.70W 173° 05.30W 173° 46.10W 172° 34.00W 170° 26.30W 172° 01.00W 169° 13.10W 168° 56.00W 167° 51.00W 166° 03.50W 171° 39.40W 165° 41.30W 171° 18.00W 171° 08.00W 165° 18.20W 167° 37.10W 170° 03.40W 168° 21.20W 165° 18.90W 165° 54.00W 166° 32.20W 167° 04.50W 165° 17.90W 166° 58.50W 169° 06.00W 1335 2135 585 447 81 61 71 67 80 84 73 93 98 117 66 111 96 75 120 126 94 104 64 129 120 69 138 113 96 107 93 70 99 70 66 66 68 101 68 107 119 85 127 103 166 97 118 129 143 114 200 1464 SI689-01 BC-15 B-5 BC-4 SI689-22 TT20-079 TT20-225 TT42-239 NW262-30 TT42-092 TT42-294 NW262-23 NW262-22 NW262-20 TT42-255 TT51-237 TT51-240 TT42-054 TT51-234 TT42-045 TT51-242 TT51-031 TT51-204 TT51-230 TT51-227 TT51-207 TT42-030 TT51-222 TT51-244 TT51-219 TT51-246 TT51-045 TT51-250 TT51-049 TT51-051 TT51-186 TT51-060 TT51-253 TT51-062 TT51-257 TT51-259 TT51-149 TT51-295 TT51-267 TT51-173 TT51-152 TT51-160 TT51-164 TT51-299 TT51-157 TT51-301 TT52-001 Productivity and biogenic flux The continental shelf of the Bering Sea covers an area of 1.2 × 106 km2 and is one of the largest in the world. The primary productivity varies between 50 and 450 gC m−2 yr−1 Copyright 2001 John Wiley & Sons, Ltd. (Antoine et al., 1996) depending upon the characteristics of the mixed layer, which are regionally controlled by the wind and the sea-ice cover (Walsh et al., 1989). A first bloom, called sea-ice edge bloom, develops along the thawing icepack during spring, in April–May (Niebauer et al., 1990, J. Quaternary Sci., Vol. 16(7) 667–680 (2001) 670 JOURNAL OF QUATERNARY SCIENCE Table 2 Sea-surface temperature and salinity around the sampling sites (NODC, 1994), sea-ice cover with more than 50% of concentration, productivity (Antoine et al., 1996), dinocyst counts and concentrations Location Site number Chukchi Sea Bering Sea Temperature February (° C) Temperature August (° C) Salinity August Sea-ice cover (months yr−1 ) Annual productivity (gC cm−2 ) Dinocyst count Dinocyst concentration (cysts cm−3 ) SI689-01 BC-15 B-5 BC-4 SI689-22 TT20-079 −1.9 −1.7 −1.6 −1.6 −1.4 −1.7 −1 −0.7 0 1 2.4 6.6 27.3 27.9 27.7 27.3 26.7 30 12 12 11.8 11.7 11.1 6.9 — 132.8 132.3 156.4 140.8 248.7 25 21 100 144 355 352 271 179 797 5459 23 843 11 548 TT20-225 TT42-239 NW262-30 TT42-092 TT42-294 NW262-23 NW262-22 NW262-20 TT42-255 TT51-237 TT51-240 TT42-054 TT51-234 TT42-045 TT51-242 TT51-031 TT51-204 TT51-230 TT51-227 TT51-207 TT42-030 TT51-222 TT51-244 TT51-219 −1.7 −1.6 −1.3 −1.3 −1.4 −0.9 −0.9 −0.2 −1.5 −1.2 −1.3 −1.3 −1 0.1 −1.5 −1.4 −1.7 −0.8 −1.5 −1.7 0.5 −0.7 −1.4 −1.4 8.2 8.4 8.5 8.5 8.4 8.7 8.7 9.1 8.5 9.2 9.1 8.6 9.2 9.2 8.5 9.5 9.1 9.3 9.5 9.1 9.3 9.9 10.2 10.7 31.5 31.2 31.7 31.7 31.6 31.7 31.7 32 31.5 32.3 32.2 31.7 32.5 32.3 31.6 32.2 31.3 32.3 32.9 31.4 31.7 31.9 32.1 31.9 4.5 5.1 5.1 4.5 4.5 4.5 3.4 4 4.5 3.5 3.6 3.8 3.5 2.3 2.6 3.6 3.9 2.5 2.6 2.9 2.5 2.6 2.8 2.6 130.9 133.9 108.2 104.5 98.9 159.9 101.8 101.6 162.6 97 116.1 183.5 97.3 100.7 84 76.4 131.6 110.5 107.8 116.3 83.02 99.6 88.4 98.9 307 125 331 305 150 341 309 306 347 333 363 328 316 323 358 347 320 331 309 302 312 335 325 269 9845 7100 8497 6191 11 608 12 018 10 853 7166 8823 6898 2356 12 162 4718 4587 5163 9802 6968 2834 6221 8507 7785 11 294 12 006 5767 TT51-246 TT51-045 TT51-250 TT51-049 TT51-051 TT51-186 TT51-060 TT51-253 TT51-062 TT51-257 TT51-259 TT51-149 TT51-295 TT51-267 TT51-173 TT51-152 TT51-160 TT51-164 TT51-299 TT51-157 TT51-301 TT52-001 −1.4 −1 −0.9 −1.3 −1.6 −1 0.3 — 1.4 2.1 2.8 2.9 2.1 3.1 2.7 2.9 2.3 3.2 3.3 3.5 3.4 3.6 10.3 8.5 9.6 8.6 8.6 8.4 9.5 10.1 9.5 9.4 8.9 9.5 9.4 8 9 9.4 8.9 9.3 9.1 8.7 8.6 8.1 32.2 31.7 32.2 31.4 31.5 31.4 31.6 32.1 31.6 32.1 32.1 31.6 31.9 32.1 32.1 31.7 32.2 32.1 32.3 32.1 32.5 32.8 2.8 2.8 1.3 2.7 2.6 2.2 1.8 1.3 1.1 0.3 0.3 0.2 0.6 0.1 0 0.2 0.2 0 0 0 0 0 100.9 216.9 128.4 159.6 192 183.2 175.8 103.8 180.7 112.7 106.8 143.3 121.5 107.6 135.2 131.1 123.6 126.3 136.4 131.3 122.6 98.9 361 327 332 334 210 308 341 245 333 329 329 384 314 316 69 339 348 462 323 447 341 317 19 024 4774 7118 5056 3243 6520 5957 8305 4143 7019 7323 5768 19 725 19 967 439 7640 16 165 27 592 17 076 11 972 5890 4263 1995; Grebmeier, 1993; Stabeno et al., 1998). This bloom, dominated by diatoms (e.g. Walsh et al., 1989; Fukuchi et al., 1993), represents approximately 65% of the annual primary production according to measurements made in the late 1970s (Niebauer et al., 1990). During the sea-ice edge Copyright 2001 John Wiley & Sons, Ltd. bloom, chlorophyll concentrations reach 3 to 5 mg m−2 , i.e. 3.6 gC m−2 day−1 (Codispoti et al., 1982; Maynard and Clark, 1987). The remaining part of the vegetative season is characterised by a decrease in nutrients and reduced phytoplankton growth (Maynard and Clark, 1987; Hanssel J. Quaternary Sci., Vol. 16(7) 667–680 (2001) DINOCYSTS IN SURFACE SEDIMENT, BERING AND CHUKCHI SEAS 671 Bering Strait Chukchi Sea r Siberia Gulf of Anadyr Yukon Anady dyr Ana it Stra AW Alaska St. Lawrence Shpanberg Strait Island ACW m 200 0m 100 Nunivak Island Bering Sea BSW s nd la n Is ia ut e Al Figure 2 Schematic illustration of the surface water circulation in the Bering Sea: AW, Anadyr Water; BSW, Bering Shelf Water; ACW, Alaskan Coastal Water et al., 1993) with a productivity of about 0.5 gC m−2 day−1 , the flora being dominated by flagellates and small diatoms (Springer and McRoy, 1993). Method Sampling and laboratory procedures Surface sediment samples analysed in the present study were taken with a gravity corer, with the exception of samples B4, B5 and B15, which were collected with a box corer (see Table 1). Sediments for palynological analyses were subsampled in the upper few centimetres (between 0 and 3 cm) of material recovered in the gravity and box corers. This interval is thought to cover the biological mixing zone and is considered representative of recent sedimentation. Depending upon the sedimentation and biological mixing rates, however, the subsampling interval may represent hundreds to thousand of years of sediment accumulation. The samples were prepared for palynological analyses following the standard procedure of the micropaleontological laboratory of GEOTOP (de Vernal et al., 1999). A microwave digestion technique adapted to the palynological preparation of Quaternary marine sediments also was used (cf. Loucheur, 1999). This technique proved extremely useful for preparing sediment samples from the Bering and Chukchi seas, which contain abundant silica particles. Palynomorphs were identified and counted using a transmitted light microscope at 250× to 1000× magnification. More than 300 dinocysts were counted in each sample for calculation of percentages and statistical analyses, with the exception of a few samples containing a sparse palynoflora (Table 2). The concentrations of palynomorphs were evaluated using the marker-grains method (Matthews, 1969), which yields results accurate to about 10% for a 0.95 confidence interval (de Vernal et al., 1987). Copyright 2001 John Wiley & Sons, Ltd. Taxonomy of the dinocyst taxa from Bering and Chukchi seas The taxonomical nomenclature used in this study conforms with that presented in Rochon et al. (1999), with a few exceptions. Cysts designated previously as Algidasphaeridium? minutum var. minutum and var. cezare are now called Islandinium minutum and Islandinium? cezare, respectively, following the systematics proposed by Head et al. (this issue). Two additional taxa also were distinguished and counted. They include the cyst of Polykrikos kofoidii and an arctic morphotype of Operculodinium centrocarpum, which are described briefly below (see also de Vernal et al., this issue). The cyst of Polykrikos kofoidii recovered in Bering Sea is characterised by a fibrous ectophragm, with extensions forming fan-shaped processes that are not linked distally (see 1–6 in Fig. 3), unlike the specimens of the cyst of Polykrikos schwartzii Bütschli 1873 as illustrated in Rochon et al. (1999). The cyst of Polykrikos kofoidii from the Bering Sea differs from Polykrikos kofoidii Chatton 1914 described by MoreyGrains and Ruse (1980) with respect to the morphology of the processes, which are fan-shaped rather than tubular. The cysts from surface sediments of Bering Sea are similar to those described by Matsuoka (1987) from the Akkeshi Bay and Saroma Lake North of Japan. However, none of the specimens from the Bering Sea exhibit shelf-like or reticulate ornamentation, as observed to occur in the Japan Sea. The cysts of Polykrikos kofoidii that were recovered in the Bering Sea show some morphological variations with respect to the size and texture of the fan-shaped processes (see 1–6 of Fig. 3). They could be associated with the Type 1 cyst of Polykrikos kofoidii described by Matsuoka and Cho (2000), but differ significantly from the Types 2 and 3 in not having separate rows of lumina between the processes. They also differ from the Type 4 Polykrikos kofoidii cyst, which is characterised by a complete ornamentation network (Matsuoka and Cho, 2000). J. Quaternary Sci., Vol. 16(7) 667–680 (2001) 672 JOURNAL OF QUATERNARY SCIENCE 1 4 3 2 5 7 8 10 11 6 9 12 Figure 3 Scale bar is 10 µm. Sample number is followed by the England Finder coordinates. (1–6). Cyst of Polykrikos kofoidii: (1) TT51-295, D8/4; (2) TT51-164, W54/2; (3) TT51-164, O47; (4) TT51-164, U35/1-3; (5) TT51-164, H43/3-4; (6) TT51-164, W16/1-2; (7) Selenopemphix nephroides: TT52-001, T44/2, apical surface. (8) Selenopemphix quanta: TT51-257, X51/2, antapical view. (9) Islandinium minutum: TT51-295, M17. (10) Lejeunecysta oliva: TT51-257, U15/2, ventral surface. (11) Brigantedinium cariacoence: TT51-295, P44/4, dorsal surface. (12) Brigantedinium simplex: TT51-164, O22/3, dorsal surface In the Bering and Chukchi seas, Operculodinium centrocarpum also presents some particularity with regard to its morphology. Several morphotypes can be differentiated on the basis of the ornamentation of the cyst wall, and the density and length of the processes (Fig. 4). In general, the cysts Copyright 2001 John Wiley & Sons, Ltd. of Operculodinium centrocarpum in the sediments of the Bering and Chukchi seas are dominated by specimens with a morphology intermediate between Operculodinium centrocarpum sensu Wall & Dale 1966 and the short processes form (cf. Rochon et al., 1999). In the Bering Sea samples, the length J. Quaternary Sci., Vol. 16(7) 667–680 (2001) DINOCYSTS IN SURFACE SEDIMENT, BERING AND CHUKCHI SEAS 1 4 2 5 673 3 6 9 Figure 4 Scale bar is 10 µm. Sample number is followed by the England Finder coordinates. (1 and 2) Operculodinium centrocarpum: TT51-60, P14/4; (1) optical cross-section; (2) lateral view. (3) Operculodinium centrocarpum —short processes: TT51-149, D8/4, lateral view. (4–6) Operculodinium centrocarpum —Arctic morphotype: TT20-225, H49; (4) ventral surface; (5) dorsal surface; (6) mid-focus. (7) Spiniferites intergrade elongatus-frigidus: TT51-227, O39/3, optical section. (8 and 9) Spiniferites frigidus: (8) TT51-60, S12/1; (9) TT51-60, F55/2 ventral surface of processes ranges from 4 to 7 µm (Fig. 4), which is less than that reported for northern North Atlantic specimens (7–14 µm; Rochon et al., 1999). However, it is of note that Operculodinium centrocarpum bearing relatively short processes were reported to occur in coastal environments of the western Pacific Ocean (Matsuoka, 1987; Matsuoka et al., 1997) and the Baltic Sea (Matthiessen and Brenner, 1996; Nehring, 1997; Ellegaard, 2000). Apart from Operculodinium centrocarpum sensu Wall & Dale 1966, several specimens exhibit a morphology intermediate with that of the type cezare described by de Vernal et al. (1989) from the post-glacial Champlain Sea material. These specimens show morphology similar to that of the cyst informally called Operculodinium centrocarpum type B by de Vernal et al. (1989). They are characterised by the presence of very few processes, which are imperfectly developed and heterogeneously distributed around the cyst. Such specimens were counted as the arctic morphotype of Operculodinium centrocarpum (see also de Vernal et al., this issue). Owing to difficulties of identification at species level, some taxa were grouped together for statistical analyses. This is the case for Brigantedinium spp., which includes Brigantedinium simplex and Brigantedinium cariacoence in addition to Copyright 2001 John Wiley & Sons, Ltd. specimens with archeopyle difficult to examine. This also is the case for Spiniferites elongatus s.l., which groups Spiniferites elongatus, Spiniferites frigidus and intergrade morphotypes (cf. Rochon et al., 1999). Specimens belonging to Protoperidium, for which determination at the genus level was difficult owing to bad orientation and/or preservation, were grouped under the label Peridinioids. Source of oceanographical data The temperature and salinity data of the surface water (0 m) are from the National Oceanographic Data Center (NODC) data sets, which includes measurements made between 1930 and 1994 (NODC, 1994). The data were compiled within a circle of 30 nautical miles around the study sites, using ORACLE data base managing system. The monthly averages of temperature and salinity for the coldest and warmest months (February and August respectively) have been used because they provide information on the annual hydrographical cycle, which seems to be more of a determinant for the dinocyst distribution than seasonal or annual means (de Vernal et al., J. Quaternary Sci., Vol. 16(7) 667–680 (2001) 674 JOURNAL OF QUATERNARY SCIENCE 1994, 1997). Sea-ice-cover data were provided by the National Climate Data Center. The original data consist of 1° × 1° grid scale measurements for the 1953–1990 period. We express the sea-ice cover as the mean number of months per year with more than 50% of sea-ice coverage. Productivity data are from Antoine et al. (1996). They are estimates from satellite observation of the chlorophyll distribution. Productivity is expressed as the annual production of carbon per surface area (gC cm−2 yr−1 ). The distribution of dinocyst assemblages Concentrations Most sediment samples contain abundant dinocysts, with concentrations ranging from 2000 to 25 000 cysts cm−3 (Fig. 5). Such concentrations are comparable to those recorded along the continental margins of the North Atlantic (e.g. de Vernal et al., 1997; Rochon et al., 1999) and reflect a relatively high productivity of dinoflagellates. A slight trend of decreasing concentration, however, is observed from south to north. Samples from northernmost sites located at the edge of the permanent ice-pack in the Chukchi Sea (Si689-01, BC15 and B-5) are characterised by concentrations lower than 800 cysts cm−3 . Low cyst concentrations in the Chukchi Sea may result from a high sedimentation rate, low cyst fluxes owing to low productivity, or both. Extreme hydrographic conditions with quasi-permanent sea-ice together with low nutrient input (e.g. Walsh et al., 1989; Hansell et al., 1993; Cooper et al., 1997) may explain low productivity. The dinocyst assemblages On the whole, the dinocyst assemblages of the Bering Sea are characterised by relatively high species diversity, with more than 20 taxa recovered routinely. However, only six taxa represent more than 90% of the assemblages. They are Operculodinium centrocarpum, the cyst of Pentapharsodinium dalei, Brigantedinium spp., Spiniferites elongatus s.l., Spiniferites ramosus and the cyst of Polykrikos kofoidii (Figs 3–5). The other accompanying taxa that are commonly recovered include Impagidinium pallidum, Islandinium minutum, Lejeunecysta oliva, Selenopemphix quanta, Selenopemphix nephroides and Quinquecuspis concreta (Figs 3–5). In the Chukchi Sea, the species diversity is much lower than that of the Bering Sea. Operculodinium centrocarpum and the cyst of Pentapharsodinium dalei largely dominate the assemblages, whereas Spiniferites elongatus s.l., Brigantedinium spp., Spiniferites ramosus and Impagidinium pallidum are observed occasionally. Relationships between the assemblages and the environmental parameters The relative abundance of the main dinocyst taxa in the assemblages shows important variations with latitude, thus suggesting a direct relationship with hydrographic and climatic conditions. In general, a decrease of the species diversity is observed from south to north. Moreover, in the Bering Sea, a linear relationship can be established between the Copyright 2001 John Wiley & Sons, Ltd. percentage of Operculodinium centrocarpum and the extent of sea-ice cover, whereas an inverse relationship is established with the percentage of the cyst of Pentapharsodinium dalei (Fig. 6). It is noteworthy that such relationships seem to be valid exclusively at the regional scale of the Bering Sea because they are not in evidence when the spectra from the Chukchi Sea are included or at the scale of the northern North Atlantic (de Vernal et al., 1997; Rochon et al., 1999). Principal component analyses were performed on taxa percentages using the software of Guiot and Goeury (1996). Logarithmic transformations were carried out in order to give more weight to accompanying taxa. The analyses reveal that the first component (PC1), which explains 51.7% of the variance, is determined by the opposition between Operculodinium centrocarpum and the other taxa (Fig. 7). Principal component 1 shows a latitudinal distribution (Fig. 8) and a significant correlation with sea-surface temperature in February and the seasonal duration of sea-ice cover (Fig. 9). The second component (PC2), which explains 15.1% of the variance, is determined by an opposition between Spiniferites elongatus s.l. and the cyst of Pentapharsodinium dalei (Fig. 7). It shows some relationship with the temperature and salinity in August (Fig. 10). There are no apparent relationships between the two first components and productivity. Definition of a regional zonation The relative proportion of taxa and the principal component analyses permitted the identification of two distinct assemblages associated with two geographical zones in the Bering Sea, and one in the Chukchi Sea (Fig. 5). The first zone (I) is characterised by high concentrations of dinocysts (up to 28 000 cysts cm−3 ) and by the dominance of the cyst of Pentapharsodinium dalei and Brigantedinium spp. It also is characterised by sparse occurrence of Operculodinium centrocarpum and the absence of its Arctic morphotype. This zone corresponds to the BSW in the southern part of the Bering Sea, adjacent to the Aleutian Islands, where yearround ice-free conditions prevail. In this area, sea-surface temperatures range from 1° to 4 ° C in February and from 8° to 11 ° C in August, and the salinity fluctuates from 31.6 to 32.8. The second zone (II) is characterised by the dominance of Operculodinium centrocarpum and its Arctic morphotype, accompanied by Spiniferites elongatus s.l. and Spiniferites ramosus. Relatively high dinocyst concentrations reaching up to 20 000 cysts cm−3 , are observed in this zone. Zone II corresponds to the northern BSW and includes the sites in the AW zone near the Anadyr Gulf, where February temperatures fluctuate from −1.6° to 0.5 ° C and sea-ice cover occurs for 2–4 months yr−1 . The Chukchi Sea represents a third zone, characterised by low species diversity. The assemblages are dominated by Operculodinium centrocarpum and the cyst of Pentapharsodinium dalei. High proportions of the Arctic morphotype of Operculodinium centrocarpum are recorded. Dinocyst concentrations are low, especially to the north, near the edge of the permanent ice pack. This zone is characterised by freezing winter temperature and extensive sea-ice cover for 7 to 12 months yr−1 . During summer, in August, temperature and salinity range −1.0° to 2.4 ° C and from 26.7 to 27.9, respectively. J. Quaternary Sci., Vol. 16(7) 667–680 (2001) Copyright 2001 John Wiley & Sons, Ltd. SI689-01 BC-15 B-5 BC-4 SI689-22 TT20-079 TT20-225 TT42-239 NW262-30 TT42-092 TT42-294 NW262-23 NW262-22 NW262-20 TT42-255 TT51-237 TT51-240 TT42-054 TT51-234 TT42-045 TT51-242 TT51-031 TT51-204 TT51-230 TT51-227 TT51-207 TT42-030 TT51-222 TT51-244 TT51-219 TT51-246 TT51-045 TT51-250 TT51-049 TT51-051 TT51-186 TT51-060 TT51-253 TT51-062 TT51-257 TT51-259 TT51-149 TT51-295 TT51-267 TT51-173 TT51-152 TT51-160 TT51-164 TT51-299 TT51-157 TT51-301 TT52-001 0 40 80 0 10 20 0 40 80 0 5 10 15 0 5 1015 0 50 20 40 60 0 5 10 0 4 0 2 020 5 10 0 14000 28000 −5 0 5 −5 a p ci in Pr PC2 0 ne po om lc 50 s nt a Se pe 2 m Te o (m 12−2 0 e -ic r ve co pe b m Te Fe ru r (˚C l Sa ity ˚C ) Assemblage zone I Assemblage zone II Assemblage zone III t us t( us g Au g Au ) in e ur at y ar 4 −20 5 10 25 28 31 r r) e ur at a ye s/ h nt Figure 5 Diagram of percentages of the main dinocyst taxa. Taxa that are not represented in the diagram are those occurring in low percentages. They include Impagidinium aculeatum, Impagidinium patulum, Lejeunecysta oliva and Lejeunecysta sabrina. The concentrations of dinocysts, the principal components (PC1 and PC2) and hydrographical means for each site also are indicated South Bering Sea Bering Strait Chukchi Sea North i le 3) m m da m pu pu m /c es r r i i . s u l d a a i t i . d 6 c c s s o ta i a in ro ro 96 nt hr cre ofo cy um p. us od nt nt 1 us ua ep on s k um id no rs at l i s t p l ce pe ce ale q n a g c s o u h (D pa ix ix is riko m ty m D in m on n ap iu o iu & m um el ra ph ph sp lyk in rph in all tio nt um ni s s i u i m m m d d a e o e e n o o o iu tr ed pe pe ec f P rit rit di fP ul W cul c m in nt en gi no no qu o ife ife r ti rc su s to nd nc le le uin yst in in ga pa i a te pe arc l pe sen ys o e e p r p i S S Q C S B O O C S Im Is S C PC1 DINOCYSTS IN SURFACE SEDIMENT, BERING AND CHUKCHI SEAS 675 J. Quaternary Sci., Vol. 16(7) 667–680 (2001) JOURNAL OF QUATERNARY SCIENCE Cyst of Pentapharsodinium dalei (%) 676 Discussion: the particularities of the assemblages from the Bering and Chukchi seas 80 70 60 50 The dinocyst assemblages from the eastern Bering Sea and Chukchi Sea are composed of taxa commonly observed in the subpolar environments of the North Atlantic. These taxa include Brigantedinium spp., Operculodinium centrocarpum, the cyst of Pentapharsodinium dalei, Islandinium minutum, Spiniferites elongatus s.l., Spiniferites ramosus and Impagidinium pallidum (de Vernal et al., 1997; Rochon et al., 1999). However, the assemblages of the Bering Sea present some particularities when compared with those of Arctic and subarctic seas adjacent to the North Atlantic. 40 30 R = 0.702 20 10 0 Operculodinium centrocarpum sensu Wall & Dale 1966 (%) 80 70 1 In high-latitude settings, the presence of the cyst of Polykrikos kofoidii seems to be a feature specific to the North Pacific (Matsuoka, 1985, 1987) and Bering Sea (this study). In the northern North Atlantic, the most similar taxon is the cyst of Polykrikos kofoidii sensu reported to occur in low latitudes (e.g. Marret, 1994; Zonneveld, 1997; Zonneveld et al., 1997). This leads us to hypothesise that the two taxa are taxonomically distinct from each other, unless they represent phenotypes adapted to different ecological niches. 60 R = 0.912 50 40 30 20 10 0 0 2 4 6 8 10 12 Sea ice cover (months/year) Figure 6 Relationship between sea-ice cover and percentages of the two dominant taxa from the Bering Sea (filled circles), Operculodinium centrocarpum sensu Wall & Dale 1966 and cyst of Pentapharsodinium dalei. Percentages of these taxa in the Chukchi Sea are indicated by open circles 2 Selenopemphix nephroides and Quinquecuspis concreta also seem to record a more boreal limit in the North Pacific and Bering Sea than in the North Atlantic (Rochon et al., 1999). Such an observation suggests that temperature probably is not the most determinant parameter for the distribution of these taxa. 3 Amongst the particularities of the Bering Sea assemblages, it is worth mentioning the morphological variations of Operculodinium centrocarpum, which bears generally short PC 2 (15.1%) 60 40 Cyst of Pentapharsodinium dalei 20 Lejeunecysta sabrina Impagidinium pallidum Impagidinium aculeatum Quinquecuspis concreta Lejeunecysta oliva 0 Spiniferites ramosus Selenopemphix quanta Selenopemphix nephroides PC 1 (51.7%) Impagidinium patulum Cyst of Polykrikos kofoidii −20 Islandinium minutum Peridinioids Islandinium? cezare Brigantedinium spp. Operculodinium centrocarpum - arctic morphotype Operculodinium centrocarpum sensu Wall & Dale 1966 Spiniferites spp. −40 Spiniferites elongatus −60 −40 −30 −20 −10 0 10 20 30 40 Figure 7 Weighting of taxa according to principal components PC1 and PC2. The open diamonds represent taxa associated with an autotrophic production, and the filled diamonds represent taxa associated with an heterotrophic production Copyright 2001 John Wiley & Sons, Ltd. J. Quaternary Sci., Vol. 16(7) 667–680 (2001) DINOCYSTS IN SURFACE SEDIMENT, BERING AND CHUKCHI SEAS 677 A PC 1 +3 to +6 0 to +3 −3 to 0 −6 to −3 B PC 2 +2 to +5 0 to +2 −2 to 0 −5 to −2 Figure 8 Maps showing spatial distribution of principal components PC1 and PC2 processes (<7 µm), and the occurrence of its Arctic morphotype characterised by a reduced density of imperfectly developed processes. Such variations in the morphology of Operculodinium centrocarpum are not exclusive to the Bering Sea. Morphological variations similar to that of the Arctic morphotype were reported in a study of the post-glacial Champlain Sea (de Vernal et al., 1989), which occupied the St Lawrence lowlands from about 12 000 to 9800 yr BP during the retreat of the Laurentide ice-sheet (Gadd, 1988). A similar morphology for Operculodinium centrocarpum also is illustrated from sediments of the Beaufort Sea in the western Canadian Arctic (Harland et al., 1980), and the southern Baltic Sea (Matthiessen and Brenner, 1996; Ellegaard, 2000). As suggested from the study of the Champlain Sea sediments, the morphological variations of Operculodinium centrocarpum could represent phenotypic adaptation to low salinity conditions (e.g. de Vernal et al., 1989). Such a hypothesis would be consistent with the relative abundance of the Arctic morphotype of Operculodinium centrocarpum in the Bering and Chukchi seas, which are characterised by a salinity lower than that of the North Atlantic Ocean. However, we failed to define a relationship between the salinity and the relative proportion of the Arctic morphotype of Operculodinium Copyright 2001 John Wiley & Sons, Ltd. centrocarpum from the assemblages of the Chukchi and Bering seas. 4 In the Bering Sea, there is a clear relationship between the seasonal extent of sea-ice or the February temperature, and the percentages of the dominant taxa, Operculodinium centrocarpum and the cyst of Pentapharsodinium dalei. However, such a relationship cannot be extrapolated on the scale of the Arctic Ocean or North Atlantic (Rochon et al., 1999; de Vernal et al., this issue), pointing towards some regionalism in the distribution of dinocyst assemblages in surface sediments of the Bering Sea. 5 In many subpolar environments marked by cold conditions with seasonal sea-ice cover, dinocyst assemblages are dominated by Peridiniales, which relate to a heterotrophic production. In the subarctic seas adjacent to the North Atlantic Ocean, Brigantedinium spp. or Islandinium minutum often dominate the assemblages (e.g. de Vernal et al., 1997, this issue; Rochon et al., 1999); in the southern Indian Ocean, Selenopemphix antarctica is characteristic of circum-Antarctic environments (Marret and de Vernal, 1997). Thus, the assemblages of the northern Bering Sea and the Chukchi Sea are peculiar because they are dominated by Operculodinium centrocarpum, the cyst of PentapharJ. Quaternary Sci., Vol. 16(7) 667–680 (2001) 678 JOURNAL OF QUATERNARY SCIENCE 32 9 R = 0.838 7 5 3 Salinity August Sea-ice cover (months/year) 33 11 31 R = 0.730 30 29 28 27 1 26 4 3 10 2 1 R = 0.819 0 −1 Temperature August (°C) Temperature February (°C) 12 8 6 R = 0.845 4 2 0 −2 −6 −4 −2 0 2 4 6 PC1 Figure 9 Correlation between PC1 and the sea-surface temperature in February and sea-ice cover. Samples from Bering Sea are represented by filled circles and those from Chukchi Sea are represented by open circles. The coefficients of correlation are based on a logarithmic relationship sodinium dalei and Spiniferites spp., which are all related to an autotrophic production. Nevertheless, the dominance of Operculodinium centrocarpum allows comparison with offshore dinocyst assemblages from the central Baffin Bay (Mudie and Short, 1985; Rochon and de Vernal, 1994), where the surface water is marked by the inflow of the West Greenland Current fed by a westward branch of the North Atlantic Drift (NAD). Similarly, the co-dominance of Operculodinium centrocarpum and the cyst of Pentapharsodinium dalei in the Bering Sea assemblages allows comparison with dinocyst assemblages from the Nordic and Barents seas, where surface waters also are under the influence of northward branches of the NAD (Rochon et al., 1999; Voronina et al., this issue). As the BSW is under the influence of northern branches of the North Pacific Current (Thomson, 1981; Reed and Stabino, 1997), we are tempted to associate the dominance of Gonyaulacales in the eastern Bering Sea to the impact of current inflow from an offshore origin. Currents no doubt play a determinant role on the dispersion of nutrient and plankton populations, and ultimately on the distribution of microfossil assemblages in sediments. 6 Some species occurring frequently in modern sediment of the northern North Atlantic Ocean, such as Ataxiodinium choane, Bitectatodinium tepikiense and Nematosphaeropsis labyrinthus (e.g. Rochon et al., 1999), have not been recorded in surface sediment of the Bering Sea despite comparable temperature and salinity conditions. The differences in species composition of assemblages of the Copyright 2001 John Wiley & Sons, Ltd. −2 −3 −2 −1 0 1 2 3 4 5 PC2 Figure 10 Relationship between PC2 and sea-surface temperature and salinity in August. Samples from Bering Sea are represented by filled circles and those from Chukchi Sea are represented by open circles. The coefficients of correlation are based on a polynomial equation (order 3) Bering Sea versus that of subarctic seas of equivalent latitude in the North Atlantic may reflect some endemism owing to limited exchanges between the North Atlantic and North Pacific. The disparity in dinocyst assemblages might be explained by different phenotypic adaptations of species to the North Pacific and the North Atlantic oceans, respectively. It might be related to differences between the two oceans, notably with respect to the concentration of nutrients or the various dissolved compounds. Conclusion The present study demonstrates that the dinocyst assemblages in the Bering and Chukchi seas are closely related to hydrographic conditions on a regional scale. In particular, the sea-surface temperature and the seasonal extent of sea-ice cover seem to exert a determinant role on the regional distribution of dinocysts assemblages, which results in a latitudinal zonation. These data indicate that dinocyst assemblages could be used for the development of transfer functions with the aim of reconstructing palaeoceanographic conditions in the northern North Pacific. However, because of differences in species composition between the Bering Sea assemblages and those of high-latitude marine basins J. Quaternary Sci., Vol. 16(7) 667–680 (2001) DINOCYSTS IN SURFACE SEDIMENT, BERING AND CHUKCHI SEAS of the North Atlantic, the use of an hemispheric scale Pacific–Arctic–Atlantic dinocyst data base for a transfer function relying on extrapolation techniques should be considered with caution. Acknowledgements Special thanks are due to B. Conard from the Oregon State University for the subsampling of core tops. We are grateful to Maryse Henry and Virginie Loucheur for their help in laboratory studies. This study has been supported by the Fonds FCAR of Quebec and the Natural Science and Engineering Council (NSERC) of Canada, notably through the project Climate System History and Dynamics (CSHD). The comments by J. Matthiessen and K. Matsuoka were most helpful in preparing the revised version of the manuscript. References Aagaard K, Roach AT, Shumacher JD. 1985. On the wind-driven variability of the flow through Bering Strait. Journal of Geophysical Research 90: 7213–7221. Antoine D, André JM, Morel A. 1996. Oceanic primary production. 2. Estimation at global scale from satellite (coastal zone colour scanner) chlorophyll. Global Biogeochemical Cycles 10: 57–69. Bujak JP. 1984. Cenozoic dinoflagellate cysts and acritarchs from the Bering Sea and northern North Pacific, DSDP Leg 19. Micropaleontology 30: 180–212. Bujak JP, Matsuoka K. 1986. Taxonomic reallocation of Cenozoic dinoflagellate cysts from Japan and the Bering Sea. Palynology 10: 235–241. Cavalieri DJ, Martin S. 1994. The contribution of Alaskan, Siberian, and Canadian coastal polynyas to the cold halocline layer of the Arctic Ocean. Journal of Geophysical Research 99: 18 343–18 362. Cavalieri DJ, Parkinson CL. 1987. On the relationship between atmospheric circulation and the fluctuations in the sea ice extents of the Bering and Okhotsk seas. Journal of Geophysical Research 92: 7141–7162. Chen C-TA. 1993. Carbonate chemistry of the wintertime Bering Sea marginal ice zone. Continental Shelf Research 13: 67–87. Chen C, Wiesenburg DA, Xie L. 1997. Influence of discharge on biological production in the inner shelf : a coupled biological and physical model of the Louisiana-Texas Shelf. Journal of Marine Research 55: 293–320. Coachman LK. 1993. On the flow field in the Chirikov Basin. Continental Shelf Research 13: 481–508. Coachman LK, Aagaard K. 1988. Transports through Bering Strait : annual and interannual variability. Journal of Geophysical Research 93: 15 535–15 539. Codispoti LA, Friederich GE, Iverson RL, Hood DW. 1982. Temporal changes in the inorganic carbon system of the south-eastern Bering Sea during spring 1980. Nature 296: 242–245. Cooney RT, Coyle KO. 1982. Trophic implication of cross-shelf copepod distribution in the southeastern Bering Sea. Marine Biology 70: 187–196. Cooper LW, Whitledge TE, Grebmeier JM, Weingartner T. 1997. The nutrient, salinity, and stable oxygen isotope composition of Bering and Chukchi seas waters in and near the Bering Strait. Journal of Geophysical Research 102: 12 563–12 573. De Vernal A, Pedersen TF. 1997. Micropaleontology and palynology of core PAR87A-10: a 23,000 year record of paleoenvironmental changes in the Gulf of Alaska, northeast North Pacific. Paleoceanography 12: 821–830. De Vernal A, Larouche A, Richard PJH. 1987. Evaluation of palynomorph concentrations: do the aliquot and the marker-grain methods yield comparable results? Pollen et Spores XXIX: 291–304. De Vernal A, Goyette C, Rodrigues CG. 1989. Contribution palynostratigraphique (dinokystes, pollens et spores) à la connaissance de la mer de Champlain: coupe de Saint-Cézare, Québec. Canadian Journal of Earth Sciences 26: 2450–2464. Copyright 2001 John Wiley & Sons, Ltd. 679 De Vernal A, Turon JL, Guiot J. 1994. Dinoflagellate cyst distribution in high-latitude marine environments and quantitative reconstruction of sea-surface salinity, temperature, and seasonality. Canadian Journal of Earth Sciences 31: 48–62. De Vernal A, Rochon A, Turon JL, Matthiessen J. 1997. Organicwalled dinoflagellate cysts: palynological tracers of sea-surface conditions in middle to high latitude marine environments. GEOBIOS 30: 905–920. De Vernal A, Henry M, Bilodeau G. 1999. Technique de préparation et d’analyse en micropaléontologie. Unpublished report 3, Les Cahiers du GEOTOP, Université du Québec à Montréal; 41 pp. De Vernal A, Hillaire-Marcel C, Turon JL, Matthiessen J. 2000. Reconstruction of sea-surface temperature, salinity, and sea-ice cover in the northern North Atlantic during the last glacial maximum based on dinocyst assemblages. Canadian Journal of Earth Sciences 37: 725–750. De Vernal A, Henry M, Matthiessen J, Mudie PJ, Rochon A, Boessenkool K, Eynaud F, Grøsfjeld K, Guiot J, Hamel D, Harland R, Head MJ, Kunz-Pirrung M, Levac E, Loucheur V, Peyron O, Pospelova V, Radi T, Turon J-L, Voronina E. 2001. Dinoflagellate cyst assemblages as tracers of sea-surface conditions in the northern North Atlantic, Arctic and sub-Arctic seas: the new ‘n = 677’ data base and its application for quantitative palaeoceanographic reconstruction. Journal of Quaternary Science 16: 681–698. Ellegaard M. 2000. Variation in dinoflagellate cyst morphology under condition of changing salinity during the last 2000 years in the Limfjord, Denmark. Review of Paleobotany and Palynology 109: 65–81. Fukuchi M, Sasaki H, Matsuda O, Tanimura A, Handa N, McRoy CP. 1993. Temporal variability of particulate flux in the northern Bering Sea. Continental Shelf Research 13: 693–704. Gadd NR (ed.). 1988. The late Quaternary Development of the Champlain Sea Basin. Special Paper Geological Association of Canada, St. John’s; 35,312 pp. Grebmeier JM. 1993. Studies of pelagic–benthic coupling extended onto the Soviet continental shelf in the northern Bering and Chukchi seas. Continental Shelf Research 13: 653–668. Grebmeier JM, Cooper LW. 1995. Influence of the St. Lawrence Island polynya upon the Bering Sea benthos. Journal of Geophysical Research 100: 4439–4460. Guiot J, Goeury C. 1996. PPPbase, a software for statistical analysis of paleoecological data. Dendrochronologia 14: 295–300. Hansell DA, Whitledge TE, Goering JJ. 1993. Patterns of nitrate utilization and new production over the Bering–Chukchi Shelf. Continental Shelf Research 13: 601–627. Harland R, Reid PC, Dobell P, Norris G. 1980. Recent and sub-Recent dinoflagellate cysts from the Beaufort Sea, Canadian Arctic. Grana 19: 211–225. Head MJ, Harland R, Matthiessen J. 2001. Cold marine indicators of the late Quaternary: the new dinoflagellate cyst genus Islandinium and related morphotypes. Journal of Quaternary Science 16: 621–636. Loucheur V. 1999. Nouveau protocole de préparation des sédiments pour l’analyse palynologique avec système micro-ondes: développement méthodologique et tests de reproductibilité. MSc thesis, Université du Québec à Montréal: Montréal; 52 pp. Marret F. 1994. Distribution of dinoflagellate cysts in recent marine sediments from the east Equatorial Atlantic (Gulf of Guinea). Review of Paleobotany and Palynology 84: 1–22. Marret F, de Vernal A. 1997. Dinoflagellate cyst distribution in surface sediments of the southern Indian Ocean. Marine Micropaleontology 29: 367–392. Marret F, de Vernal A, Pedersen TF, McDonald D. 2001. Middle Pleistocene to Holocene palynostratigraphy of ODP 887 in the Golf of Alaska, Northeastern North Pacific. Canadian Journal of Earth Sciences 38: 373–386. Matsuoka K. 1985. Organic-walled dinoflagellate cysts from surface sediments of Nagasaki Bay and Senzaki Bay, West Japan. Bulletin of the Faculty of Liberal Arts, Nagasaki University 25: 21–115. Matsuoka K. 1987. Organic-walled dinoflagellate cysts from surface sediments of Akkeshi Bay and Lake Saroma, North Japan. Bulletin of the Faculty of Liberal Arts, Nagasaki University 28: 35–123. Matsuoka K. 1992. Species diversity of modern dinoflagellate cysts in surface sediments around the Japanese islands. In Neogene J. Quaternary Sci., Vol. 16(7) 667–680 (2001) 680 JOURNAL OF QUATERNARY SCIENCE and Quaternary Dinoflagellate Cysts and Acritarchs, Head MJ, Wrenn JH (eds). American Association of Stratigraphic Palynologists Foundation: Dallas; 33–53. Matsuoka K, Bujak JP. 1988. Cenozoic dinoflagellate cysts from the Navaran basin, Norton Sound and St. George basin, Bering Sea. Bulletin of the Faculty of Liberal Arts, Nagasaki University 29: 1–147. Matsuoka K, Cho HJ. 2000. Morphological variation in cyst of the gymnodinialean dinoflagellate Polykrikos. Micropaleontology 46: 360–364. Matsuoka K, McMinn A, Wrenn JH. 1997. Restudy of holotype of Operculodinium centrocarpum (Deflandre & Cookson) Wall (Dinophyceae) from the Miocene of Australia, and taxonomy of related species. Palynology 21: 19–33. Matthews J. 1969. The assessment of a method for the determination of absolute pollen frequencies. New Phytologist 68: 161–166. Matthiessen J. 1995. Distribution patterns of dinoflagellate cysts and other organic-walled microfossils in recent Norwegian-Greenland Sea sediments. Marine Micropaleontology 24: 307–334. Matthiessen J, Brenner W. 1996. Chlorococcalalgen und dinoflagellaten-zysten in rezenten sedimenten des Greifswalder Boddens (südliche Ostsee). Senkenbergiana maritima 27: 33–48. Maynard NG, Clark DK. 1987. Satellite color observations of spring blooming in Bering Sea shelf waters during the ice edge retreat in 1980. Journal of Geophysical Research 92: 7127–7139. Morey-Grains G, Ruse RH. 1980. Encystement and reproduction of the predatory dinoflagellate, Polykrikos kofoidii Chatton (Gymnodiniales). Phycologia 19: 230–236. Mudie PJ. 1992. Circum-Arctic Quaternary and Neogene marine palynoflora: paleoecology and statistical analysis. In Neogene and Quaternary Dinoflagellate Cysts and Acritarchs, Head MJ, Wrenn JH (eds). American Association of Stratigraphic Palynologists Foundation: Dallas; 347–390. Mudie PJ, Short SK. 1985. Marine palynology of Baffin Bay. In Quaternary environment, Eastern Canadian Arctic, Baffin Bay and West Greenland, Andrews JT (ed.). Allen & Unwin: Winchester; 263–308. Nehring S. 1997. Dinoflagellate resting cysts from recent German coastal sediment. Botanica marina 40: 307–324. Niebauer HJ. 1988. Effects of El-Niño–Southern Oscillation and North Pacific weather patterns on interannual variability in the subarctic Bering Sea. Journal of Geophysical Research 93: 5051–5068. Niebauer HJ, Alexander VA, Henrichs SM. 1990. Physical and biological oceanographic interaction in the spring bloom at the Bering Sea marginal ice edge zone. Journal of Geophysical Research 95: 22 229–22 242. Niebauer HJ, Alexander VA, Henrichs SM. 1995. A time-series study of the spring bloom at the Bering Sea ice edge. I. Physical processes, chlorophyll and nutrient chemistry. Continental Shelf Research 15: 1859–1877. Copyright 2001 John Wiley & Sons, Ltd. NODC. 1994; World Ocean Atlas. National Oceanic and Atmospheric Administration, Boulder, Colorado. CD-ROM data sets. Overland JE, Roach AT. 1987. Northward flow in the Bering and Chukchi seas. Journal of Geophysical Research 92: 7097–7105. Reed RK, Stabeno PJ. 1997. Long-term measurements of flow near the Aleutian Islands. Journal of Marine Research 55: 565–575. Roach AT, Aagaard K, Pease CH, Salo SA, Weingartner T, Pavlov V, Kulakov M. 1995. Direct measurements of transport and water properties through the Bering Strait. Journal of Geophysical Research 100: 18 443–18 457. Rochon A, de Vernal A. 1994. Palynomorph distribution in Recent sediments from the Labrador Sea. Canadian Journal of Earth Sciences 3: 115–127. Rochon A, de Vernal A, Turon JL, Matthiessen J, Head MJ. 1999. Distribution of Dinoflagellate Cysts in Surface Sediments from the North Atlantic Ocean and Adjacent Basins and Quantitative Reconstruction of Sea-surface Parameters. Special Contribution Series 35, American Association of Stratigraphic Palynologists Foundation: Dallas; 152 pp. Springer AM, McRoy CP. 1993. The paradox of pelagic food webs in the northern Bering Sea—III. Patterns of primary production. Continental Shelf Research 13: 575–599. Stabeno PJ, Schumacher RF, Davis RF, Napp JM. 1998. Under-ice observations of water column temperature, salinity and spring phytoplankton dynamics: eastern Bering Sea shelf. Journal of Marine Research 56: 239–255. Thomson RE. 1981; Oceanography of the British Columbia Coast. Special Publication 56, Canadian Fisheries and Aquatic Sciences: Ottawa; 291 pp. Voronina E, Polyak L, de Vernal A, Peyron O. 2001. Holocene variations of sea-surface conditions in the southeastern Barents Sea, reconstructed from dinoflagellate cyst assemblages. Journal of Quaternary Science 16: 717–726. Walsh JJ, McRoy CP. 1986. Ecosystem analysis in the southeastern Bering Sea. Continental Shelf Research 5: 259–288. Walsh JJ, McRoy CP, Coachman LK, Goering JJ, Nihoul JJ, Whitledge TE, Blackburn TH, Parker PL, Wirick CD, Shuert PG, Grebmeier JM, Springer AM, Tripp RD, Hansell DA, Djenidi S, Deleersnijder E, Henriksen K, Lund BA, Andersen P, Müller-Karger FE, Dean K. 1989. Carbon and nitrogen cycling within the Bering/Chukchi Seas: source regions for organic matter effecting AOU demands of the Arctic Ocean. Progress in Oceanography 22: 277–359. Zonneveld KAF. 1997. Dinoflagellate cysts distribution in surface sediments from the Arabian Sea (northwestern Indian Ocean) in relation to temperature and salinity gradients in the upper water column. Deep-Sea Research II: 1411–1443. Zonneveld KAF, Ganssen G, Troelstra S, Versteegh GJM, Visscher H. 1997. Mechanism forcing abrupt fluctuations of the Indian Ocean summer monsoon during the last deglaciation. Quaternary Science Reviews 16: 187–201. J. Quaternary Sci., Vol. 16(7) 667–680 (2001)