* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Syntabulin, a motor protein linker, controls dorsal
Vectors in gene therapy wikipedia , lookup
Polyadenylation wikipedia , lookup
Gene therapy of the human retina wikipedia , lookup
Gene expression programming wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
History of RNA biology wikipedia , lookup
Epigenetics in learning and memory wikipedia , lookup
Long non-coding RNA wikipedia , lookup
Preimplantation genetic diagnosis wikipedia , lookup
RNA interference wikipedia , lookup
Genomic imprinting wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
RNA silencing wikipedia , lookup
Nutriepigenomics wikipedia , lookup
Epigenetics of human development wikipedia , lookup
Gene expression profiling wikipedia , lookup
Messenger RNA wikipedia , lookup
Non-coding RNA wikipedia , lookup
Primary transcript wikipedia , lookup
Designer baby wikipedia , lookup
Epitranscriptome wikipedia , lookup
RNA-binding protein wikipedia , lookup
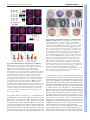
Development Advance Online Articles. First posted online on 11 February 2010 as 10.1242/dev.046425 ePressatonline publication date 11 February 2010 AccessDevelopment the most recent version http://dev.biologists.org/lookup/doi/10.1242/dev.046425 RESEARCH ARTICLE 1 Development 137, 0000-0000 (2010) doi:10.1242/dev.046425 © 2010. Published by The Company of Biologists Ltd Syntabulin, a motor protein linker, controls dorsal determination Hideaki Nojima1, Sophie Rothhämel2, Takashi Shimizu1, Cheol-Hee Kim3, Shigenobu Yonemura4, Florence L. Marlow2 and Masahiko Hibi1,5,* SUMMARY In amphibian and teleost embryos, the dorsal determinants (DDs) are believed to be initially localized to the vegetal pole and then transported to the prospective dorsal side of the embryo along a microtubule array. The DDs are known to activate the canonical Wnt pathway and thereby promote the expression of genes that induce the dorsal organizer. Here, by identifying the locus of the maternal-effect ventralized mutant tokkaebi, we show that Syntabulin, a linker of the kinesin I motor protein, is essential for dorsal determination in zebrafish. We found that syntabulin mRNA is transported to the vegetal pole during oogenesis through the Bucky ball (Buc)-mediated Balbiani body-dependent pathway, which is necessary for establishment of animal-vegetal (AV) oocyte polarity. We demonstrate that Syntabulin is translocated from the vegetal pole in a microtubule-dependent manner. Our findings suggest that Syntabulin regulates the microtubule-dependent transport of the DDs, and provide evidence for the link between AV and dorsoventral axis formation. INTRODUCTION During early vertebrate embryogenesis, the dorsal organizer plays an important role in the formation of the dorsoventral (DV) and anteroposterior (AP) body axes. The molecular mechanisms that control the zygotic gene cascades that induce the organizer have been elucidated (De Robertis, 2006; De Robertis et al., 2000; Gerhart et al., 1989; Hibi et al., 2002; Schier and Talbot, 2005). However, the mechanisms that initially specify the dorsal axis and induce zygotic genes that are required for dorsal organizer formation are not fully understood. In amphibian and teleost embryos, specification of the dorsal axis begins soon after fertilization. In Xenopus, the egg cortex rotates relative to the sperm entry point during the first cell cycle (cortical rotation). During this process, small particles and organelles are transported along a microtubule array that is oriented with the plus ends towards the prospective dorsal side (Elinson and Rowning, 1988; Houliston and Elinson, 1991). In zebrafish, the sperm enters at the animal pole. A parallel microtubule array forms at the vegetal pole of the yolk ~20 minutes post-fertilization (mpf) (Jesuthasan and Stahle, 1997). Disruption of the microtubule array by treatment with nocodazol, cold or UV irradiation prior to the 32-cell stage leads to loss of the dorsal organizer and to the ventralization of embryos (Jesuthasan and Stahle, 1997). Removal of the vegetal yolk mass at the early 1-cell stage also results in severely ventralized embryos (Mizuno et al., 1999; Ober and Schulte-Merker, 1999). These studies established the hypothesis that the dorsal determinants (DDs) are initially 1 Laboratory for Vertebrate Axis Formation, RIKEN Center for Developmental Biology, Hyogo 650-0047, Japan. 2Department of Developmental and Molecular Biology, Albert Einstein College of Medicine, New York, NY 10461, USA. 3Department of Biological Sciences and GRAST, Chungnam National University, Daejeion 305-764, Korea. 4Electron Microscope Laboratory, RIKEN Center for Developmental Biology, Hyogo 650-0047, Japan. 5Bioscience and Biotechnology Center, Nagoya University, Nagoya 464-8601, Japan. *Author for correspondence ([email protected]) Accepted 12 January 2010 localized to the vegetal pole and then transported along microtubules to the prospective dorsal side, where they are incorporated by dorsal blastomeres and promote dorsal organizer formation (see Fig. S1 in the supplementary material). However, the molecular identity of the DDs, and the mechanisms that localize them to the vegetal pole and mediate their subsequent transport to the prospective dorsal blastomeres, remain unknown. Although the molecular nature of the DDs is not clear, they are known to activate the canonical Wnt pathway, which leads to nuclear accumulation of b-catenin. Activation of the canonical Wnt pathway in Xenopus and zebrafish embryos results in ectopic formation or expansion of the dorsal organizer, whereas its inhibition impairs dorsal axis formation and reduces dorsal-specific gene expression (reviewed by Hibi et al., 2002). These data place canonical Wnt pathway function downstream of the DDs. In zebrafish, the b-catenin accumulates in the nuclei of dorsal blastomeres by the 128-cell stage and in the dorsal blastoderm and dorsal yolk syncytial layer of mid-blastula stage embryos (Dougan et al., 2003; Schneider et al., 1996). Thus, the DDs activate canonical Wnt signaling in the dorsal blastomeres by the 128-cell stage. A bcatenin and Tcf/Lef complex is thought to regulate zygotic expression of dorsal-specific genes, including the homeobox gene dharma (dha, also known as bozozok) and the nodal-related gene ndr1 (also known as squint) (Dougan et al., 2003; Leung et al., 2003; Ryu et al., 2001; Shimizu et al., 2000a), which function in dorsal organizer formation (Shimizu et al., 2000a; Sirotkin et al., 2000). Thus, the DDs induce the expression of these dorsal-specific genes through activation of the canonical Wnt pathway and thereby promote dorsal organizer formation. Genetic studies in zebrafish have revealed maternal factors that are involved in dorsal determination. The recessive maternal-effect mutant ichabod shows impaired maternal expression of b-catenin 2 (ctnnb2; one of two b-catenin genes in zebrafish), defective dorsal organizer formation and severe ventralization (Bellipanni et al., 2006; Kelly et al., 2000). The maternal-effect mutant brom bones, which has a nonsense mutation disrupting hnRNP I (ptbp1a – DEVELOPMENT KEY WORDS: Dorsal determination, Syntabulin, Kinesin, Microtubules, Balbiani body, Bucky ball, Zebrafish RESEARCH ARTICLE Zebrafish Information Network), shows egg activation defects, disorganized vegetal microtubule array formation, and subsequently displays ventralization (Mei et al., 2009). Furthermore, a ubiquitin ligase, tripartite motif-containing 36 (trim36), is localized to the vegetal cortex of Xenopus oocytes, and when depleted causes defective vegetal microtubule array formation and ventralization (Cuykendall and Houston, 2009). Cumulatively, these data support the hypothesis that microtubule-dependent transport of DDs plays an essential role in canonical Wnt pathway activation and dorsal axis determination in teleost and amphibian embryos. The DDs are present at the vegetal pole of eggs prior to fertilization and therefore achieve their vegetal pole localization during oogenesis. In Xenopus and zebrafish oocytes, mRNAs localize to the oocyte vegetal pole via overlapping transport pathways. The early pathway involves recruitment of mRNAs, including germline-specific transcripts to the Balbiani body (Kloc et al., 2001; Kloc and Etkin, 1995; Kloc et al., 1996; Wilk et al., 2005), an evolutionarily conserved aggregate of organelles and molecules that is present in early oocytes (Guraya, 1979; Kloc et al., 2004). Balbiani body formation is controlled by Bucky ball (Buc). Oocytes lacking Buc function fail to localize mRNAs to the vegetal pole in early and late stage oocytes, and show defective animalvegetal (AV) polarity (Bontems et al., 2009; Marlow and Mullins, 2008). However, the relationship between the Balbiani bodydependent RNA transport system and vegetal pole localization of the DDs has not been fully elucidated. The zebrafish maternal-effect recessive mutation tokkaebi (tkk) affects the formation of dorsal structures (Nojima et al., 2004). Embryos obtained from tkk homozygous females (tkk embryos) show severely ventralized phenotypes. Accumulation of b-catenin in the dorsal nuclei at mid-blastula stages is impaired in tkk embryos, indicating that tkk functions upstream of activation or stabilization of b-catenin in the maternal Wnt signaling pathway. In this report, we show that the tkk locus encodes a zebrafish ortholog of Syntabulin, a linker molecule that attaches cargo to the motor protein kinesin I in neuronal axons (Cai et al., 2005; Cai et al., 2007; Su et al., 2004). We demonstrate that syntabulin RNA is transported to the vegetal pole during oogenesis through the Buc-mediated Balbiani body-dependent pathway. We further show that Syntabulin protein translocates from the vegetal pole in a microtubule-dependent manner during early embryogenesis. Our findings suggest that Syntabulin is involved in the vegetal pole localization and microtubule-dependent transport of the DDs, and that Syntabulin links oocyte AV polarity and embryonic DV polarity. MATERIALS AND METHODS Positional cloning tkkrk4 homozygous fish were crossed with wild-type India fish (Knapik et al., 1996) to generate F1 families. Homozygous tkkrk4 female fish were raised from the F1 cross and identified based on the ventralized phenotypes of their offspring. Since embryos from tkkrk4/rk4 female fish with India genetic background show relatively mild phenotypes and low penetrance (Nojima et al., 2004), phenotypic analysis was performed at least twice. Genomic DNA from mutant females was used to carry out segregation analyses with SSLP markers. z1543 on chromosome 16 was found to be close to the tkk locus (one recombination event out of 280 meiosis). Using the PCR primers for z1543 as a probe, BAC DKEY-97P23 was obtained. Through BAC end sequencing and rescreening of BAC clones, seven BAC clones (DKEY-22J10, 42F24, 9G17, 96G15, 71M14, 106H17, 97P23) were found to cover the tkk locus (see Fig. 2A). Further linkage analyses revealed that four BAC clones (DKEY-42F24, 9G17, 96G15, 71M14) contained the tkk locus. The inserts in these BAC clones were sequenced by a shotgun sequencing procedure and 12 genes were found in this region. The sequence Development 137 (6) and gene information in the region were also confirmed by the current zebrafish genome data (http://www.sanger.ac.uk/Projects/D_rerio/). Expression of the 12 candidate genes in ovary or in 1-cell stage embryos was analyzed by RT-PCR (see Table S1 in the supplementary material). The cDNAs of syntabulin and nine other maternally expressed candidates were isolated from wild-type and tkk 1-cell stage embryos by RT-PCR and cloned into pBluescript II SK+ (Stratagene). A 3⬘ terminal fragment of the insertion in the syntabulin promoter was isolated by inverse PCR with primers 5⬘AGTATTTAATTCTGAACAAACAGAAC-3⬘ and 5⬘-GTGTGTTATGTAATATGCATTTTTAC-3⬘, as described (Kawakami et al., 2004). The insertion was detected by PCR with primer1, 5⬘-GATCCATTCCCAACTTTATTTTCTCG-3⬘ and primer2, 5⬘-AGTATTTAATTCTGAACAAACAGAAC-3⬘ (see Fig. 2G). The cDNA, genomic and insert sequences of zebrafish syntabulin were deposited in the DDBJ databank under the accession numbers AB517946, AB517947 and AB517948, respectively. For phenotypic analysis, tkk embryos were obtained from crosses of young (3 month old) homozygous male and female fish in the TL genetic background (Nojima et al., 2004). For time course analysis, in vitro fertilization was performed (Westerfield, 1995). For studies of bucky ball mutants, bucp106re (Dosch et al., 2004; Marlow and Mullins, 2008) and bucp43bmtb (Bontems et al., 2009) alleles were used. RT-PCR Quantitative (q) RT-PCR analysis was conducted to examine the expression of syntabulin, the other candidate genes and Wnt genes in wild-type and tkk embryos, using an ABI Prism 7000 (Applied Biosystems) or LightCycler 480 (Roche) with Power SYBR Green PCR Master Mix (Applied Biosystems) or SYBR Green I Master (Roche), respectively. Primers used were: syntabulin, 5⬘-ATGGAGTTGGCGCAAAATG-3⬘ and 5⬘TCTTCGCCTCGGTAGAACTTTC-3⬘; zdhhc3, 5⬘-ATGAGCGTCAGAACAGCATGTG-3⬘ and 5⬘-AAGACGAGAAACCAGGTGATGATC-3⬘; e(y)2, 5⬘-GTTGCTGGAGTTACTCCCAAAGG-3⬘ and 5⬘-GCGAGAAAGGCTCTTATTCTCTGA-3⬘; gapdh, 5⬘-GGATCTGACAGTCCGTCTTGAGA-3⬘ and 5⬘-TGCAGCCTTGACGACTTTCTT-3⬘; for primer information for the other candidate genes, see Table S2 in the supplementary material. All PCR assays were performed in triplicate. The relative mRNA level of the averaged CT (cycle threshold, ABI Prism 7000) or CP (crossing point, LightCycler 480) was quantified and normalized to gapdh. For detection of syntabulin in wild-type and buc mutant oocytes (see Fig. 6), ovaries were dissected from AB, bucp106re and bucp43bmtb mutant female fish. Fifty stage I/II or III oocytes were manually sorted for RNA isolation and cDNA preparation. qRT-PCR was carried out using the Realplex2 System (Eppendorf) with Power SYBR Green PCR Master Mix. Primers used were: syntabulin, 5⬘-CACACTGATGCTGTGCTGAA-3⬘ and 5⬘-TCCTGCGAGTGAATGAGAGA-3⬘; ef1a, 5⬘- AGCCTGGTATGGTTGTGACCTTCG3⬘ and 5⬘-CCAAGTTGTTTTCCTTTCCTGCG-3⬘. The relative mRNA level of the averaged CT was quantified and normalized to ef1a. In situ hybridization Whole-mount in situ hybridization was performed as described previously (Thisse and Thisse, 1998) except that hybridization was at 65°C. BM purple AP substrate (Roche) was used as the substrate for alkaline phosphatase. dharma (bozozok) (Yamanaka et al., 1998) and dusp6 (mkp3) detection was as described previously (Tsang et al., 2004). For the syntabulin probe, the 3⬘UTR of syntabulin was amplified by PCR using the primers 5¢GCTCTAGATAGCGGCCCTCACCTACTTCTACAAC-3¢ and 5¢-ACGCGTCGACTTTTCCATGCTTTAATAATAAATATA-3¢. The BM purple signals were acquired using an AxioPlan2 or AxioSkop2 microscope equipped with an AxioCam CCD camera (Zeiss), or using an Olympus SZ16 fluorescence dissecting microscope with a Microfire digital camera (Olympus). Images were processed in Photoshop (Adobe), Illustrator (Adobe) and Canvas (ACD systems). In situ hybridization on slides was essentially as described previously (Marlow and Mullins, 2008). To obtain eggs, wild-type and mutant females were anesthetized in Tricaine as described (Westerfield, 1995). Eggs were activated by adding embryo medium, and then fixed with 4% paraformaldehyde (PFA) in phosphatebuffered saline (PBS) 2 or 5 minutes after activation. DEVELOPMENT 2 Dorsal termination by a motor protein linker Immunostaining For b-catenin and Syntabulin staining, embryos were fixed with 4% PFA in PBS for 24 hours at 4°C. For microtubule staining, embryos were fixed with a microtubule-stabilizing buffer: 80 mM potassium Pipes pH 6.8, 5 mM EGTA, 1 mM MgCl2, 3.7% formaldehyde, 0.25% glutaraldehyde, 0.2% Triton X-100 (Schroeder and Gard, 1992). The dechorionated embryos were washed with PBS containing 0.1% Triton X-100 (PBS-T) and blocked with 1% bovine serum albumin (BSA) in PBS-T for 1 hour. Detection of bcatenin was as described previously (Nojima et al., 2004), and the immune complexes were visualized with diaminobenzidine (DAB, Sigma). For detection of Syntabulin, the samples were incubated with anti-Syntabulin antibody solution (anti-Syn1, 1/50 dilution of hybridoma supernatant; antiSyn2, 1/500 of hybridoma supernatant; anti-SynP, 1/500 of affinity-purified antibody) at 4°C overnight. For detection of microtubules, the samples were incubated with mouse monoclonal anti-b-tubulin antibody solution (1/1000, Chemicon KMX-1). After four washes with PBS-T, the samples were incubated with secondary antibodies [1/500, Alexa Fluor 555 goat antimouse or goat anti-rabbit IgG (H+L), Molecular Probes]. The DAB signals (b-catenin) and fluorescent images (Syntabulin) were obtained by AxioPlan2 imaging. Microtubule images were taken with an LSM5 Pascal laser-scanning inverted microscope (Carl Zeiss) and constructed from z-stack sections by a 3D projection program associated with the microscope. RESEARCH ARTICLE 3 HRP-conjugated rabbit anti-rat IgG (H+L) (Zymed) or HRP-conjugated anti-mouse IgG (Mouse TrueBlot, eBioscience) antibody, using a chemiluminescence system (Western Lightning, Perkin Elmer Life Science). For detection of Syntabulin in embryos, 200 embryos were lysed in 700 ml of lysis buffer by grinding on ice. The lysates were cleared by centrifugation and immunoprecipitated with anti-Syntabulin antibodies (1 ml of ascites of monoclonal antibodies). Treatment with microtubule inhibitors Stock solutions of nocodazole (Sigma; 2 mg/ml in DMSO) and colchicine (Sigma; 100 mg/ml in ethanol) were diluted to 1 mg/ml or 0.4 mg/ml, respectively, in embryo medium (Westerfield, 1995). Embryos were placed in inhibitor solutions with their chorion intact, just after in vitro fertilization, for the times indicated. Electron microscopy Electron microscopy (see Fig. S2 in the supplementary material) was carried out essentially as described previously (Shimizu et al., 2005; Yonemura et al., 2002). Statistics Statistical analyses were performed by two-tailed Student’s t-test using GraphPad Prism 5. P<0.01 was considered significant. The Tol2 rescue plasmids were constructed using Gateway technology (Invitrogen) in pT2KDest-RfaF, which is derived from pT2KXIG (Kawakami et al., 2004). Further information about the plasmids is available on request. The promoter of zona pellucida protein C (zpc), which is derived from zp0.5GFP, was described previously (Onichtchouk et al., 2003). The polyadenylation signal (pAS) is derived from pCS2+. GFP refers to mmGFP5 derived from zp0.5GFP. Venus is a modified YFP and is derived from pCS2+venus (Nagai et al., 2002). The 2A peptide sequence is derived from porcine teschovirus-1 (PTV1) (Provost et al., 2007) and the DNA fragment is generated with a synthesized oligonucleotide: 5⬘GTCGACGGATCAGGCGCTACGAATTTCTCGCTACTGAAGCAGGCTGGAGATGTAGAGGAAAACCCGGGACCGGCCATGG-3⬘(linkers are underlined), which corresponds to GSGATNFSLLKQAGDVEENPGP. To connect syntabulin and Venus with the 2A peptide sequence, the stop codon of syntabulin was mutated to introduce a SalI site. The 2A peptide sequence and Venus were linked at an NcoI site. To make transgenic fish, 25 pg of Tol2 transgene plasmid DNA and 25 pg of transposase RNA were injected into 1-cell stage embryos obtained from crosses of wild-type or relatively old tkk pairs. Expression plasmids for 6⫻Myc-tagged Syntabulin and 3⫻HAtagged Kif5b were constructed in pCS2+. Antibodies To raise monoclonal and polyclonal antibodies against Syntabulin, glutathione S-transferase (GST) fusion proteins containing amino acids 1498 (Syn1 antigen) or amino acids 102-271 (Syn2 antigen) of Syntabulin, or a His-tagged protein containing amino acids 1-120 of Syntabulin were generated in E. coli. The GST fusion and His-tagged proteins were purified and used for immunization. For generating monoclonal antibodies, BALB/c mice were immunized four or five times with ~50 mg of the proteins. Spleen cells of the immunized mice were fused with the mouse myeloma line Ag8.563, and hybridomas were obtained by conventional HAT selection. Supernatants of growth-positive cells were subjected to enzyme-liked immunosorbent assays (ELISAs) to identify hybridomas producing specific antibodies. A polyclonal antibody (anti-SynP) was generated by immunizing rabbits with the His-tagged protein six times. The antibody was purified using an affinity column that was covalently conjugated to the antigen. Transfection, immunoprecipitation and immunoblotting HEK293T cells in a 6-cm dish were transfected using HilyMax (DOJINDO Laboratories). The cells were lysed at 24 hours post-transfection in 1 ml of lysis buffer: 10 mM HEPES-HCl pH 7.4, 150 mM NaCl, 1% NP40, 1 mM EDTA, protease inhibitor cocktail (Nacalai). The lysates were precipitated with anti-HA antibody (3F10, Roche), resolved by 5-20% SDS-PAGE (SuperSep, Wako, Japan), and blotted with either anti-HA (3F10, Roche) or anti-Myc (9E10, SantaCruz) antibodies. The proteins were detected with an RESULTS Positional cloning of the tokkaebi gene reveals a novel role for Syntabulin in dorsal axis formation tokkaebi (tkk) is a maternal-effect recessive mutation that disrupts a gene required for dorsal axis formation in zebrafish. The progeny of tkk homozygous females (tkk embryos) display severely ventralized phenotypes, which are more severe in the TL genetic background (Nojima et al., 2004). We reassessed the expression of the earliest known zygotic dorsal-specific genes and nuclear accumulation of bcatenin in tkk embryos obtained after backcrossing with TL fish. Approximately 80% of tkk embryos from young females showed complete ventralization at the pharyngula stage [24 hours postfertilization (hpf); class C1, Fig. 1A]. In tkk embryos, expression of the earliest known dorsal-specific genes dharma (dha) and dualspecificity phosphatase 6 [dusp6, also known as mitogen-activated protein kinase phosphatase 3 (mkp3)], two direct targets of the canonical Wnt pathway (Ryu et al., 2001; Tsang et al., 2004; Yamanaka et al., 1998), was absent or strongly reduced at mid-tolate blastula stage (4 hpf, Fig. 1H-K). Dorsal accumulation of bcatenin at the 256-cell stage was observed in wild-type, but not in tkk, embryos (Fig. 1F,G). A parallel microtubule array that is thought to play an important role in dorsal determination (Jesuthasan and Stahle, 1997) was present at the vegetal pole at 20 mpf in wild-type (Fig. 1L) and in tkk (Fig. 1M) embryos. Electron microscopy revealed a similar composition of organelles, including ribosomes at the vegetal pole, of wild-type and tkk embryos at 20 mpf (see Fig. S2 in the supplementary material), indicating that the vegetal yolk is not significantly affected in tkk embryos. Together, these data suggest that the tkk gene product is dispensable for vegetal pole microtubule formation, but functions in early embryogenesis or oogenesis to promote nuclear accumulation of b-catenin and expression of zygotic genes required for dorsal organizer formation, including dha. To reveal how the tkk gene product contributes to dorsal determination, we carried out positional cloning. We mapped the tkk locus to a 600 kb region of chromosome 16 that contains 12 potential open reading frames (Fig. 2A). Although ten of the 12 genes were maternally expressed, none had a nonsense or missense mutation in their coding region (see Table S1 in the supplementary material for sequence information; data not shown). However, using qRT-PCR DEVELOPMENT Plasmid construction and transgenesis 4 RESEARCH ARTICLE Development 137 (6) genome (Fig. 2G and see Fig. S5 in the supplementary material). The 3⬘ sequence of the insertion shows similarities to various genome regions and to the pol-like protein of snail and Drosophila retrotransposons (accession numbers ABN58714, AAA70222) at the amino acid level, implying that the insertion is a transposable element. Our expression data suggest that this insertion in the promoter region strongly suppresses transcription of the syntabulin gene. analysis, we found that the maternal expression of one candidate gene was strongly reduced in tkk embryos (Fig. 2B and see Table S1 in the supplementary material). The gene encodes the zebrafish ortholog of Syntabulin (Su et al., 2004), based on strong sequence similarity to human syntabulin (see Fig. S3 in the supplementary material) and on synteny between the tkk chromosomal region in zebrafish and the human syntabulin chromosomal region (chromosome 8, data not shown). Expression of zebrafish syntabulin remained strongly reduced at 24 hpf in tkk as compared with wild-type embryos (see Fig. S4 in the supplementary material). Whole-mount in situ hybridization revealed vegetal pole localization of syntabulin mRNA in wild-type eggs and embryos through the 16-cell stage (Fig. 2C-E see Fig. S4 in the supplementary material), and its absence in tkk eggs and embryos (Fig. 2F). By genomic PCR and Southern blot analyses, we found an insertion in the syntabulin promoter region (232 bp upstream of the transcription initiation site) in the tkk mutant Exon-intron structure of syntabulin is required for vegetal pole localization and proper dorsal determination The 3⬘ UTR of germline-specific mRNAs, such as vasa, nanos and dazl, is necessary and sufficient for their vegetal pole localization (Kosaka et al., 2007). In tkk embryos rescued by maternal expression of syntabulin mRNA containing the first intron and 3⬘ UTR, the mRNA was not vegetally restricted, but was instead distributed throughout the embryos (Fig. 3E,F). Furthermore, EGFP-3⬘ UTR RNA was not localized to the vegetal pole in transgenic wild-type or tkk zygote-stage embryos (Fig. 4A-E). These data suggest that the 3⬘ UTR of syntabulin is not sufficient for its vegetal pole localization. In Drosophila oocytes, oskar mRNA localization at the posterior pole, which is required for oogenesis, is controlled in part by splicing (Hachet and Ephrussi, 2004). Therefore, we examined whether the exon-intron structure of the syntabulin gene is required for the vegetal localization of DEVELOPMENT Fig. 1. Maternal-effect tokkaebi (tkk) mutant zebrafish embryos show defects in dorsal determination. (A-E) Phenotypes of tkk embryos (A-D) versus wild type (WT; E) at 24 hpf. Lateral views with dorsal to the right (A-D) or to the top (E). Embryos were classified into four classes (C1-4) based on their ventralized phenotypes. C1 embryos displayed a completely ventralized phenotype. C2 embryos were similar to C1 embryos but had somites in the trunk region. C3 embryos had somites and spinal cord in the trunk. C4 embryos had somites, spinal cord and hindbrain, but lacked notochord or neuroectoderm anterior to the midbrain. The percentage of tkk embryos from typical young females that showed each phenotype is indicated. (F,G) Immunostaining of b-catenin at the 256-cell stage in wild-type (F; 88%, n=26) and tkk (G; 11%, n=35) embryos. Dorsal views. Arrows indicate nuclear accumulation of b-catenin in dorsal blastomeres. (H,I) Expression of dha at sphere stage (4 hpf) in wild-type (H; 100%, n=36) and tkk (I; 8%, n=40) embryos. (J,K) Expression of dusp6 at sphere stage in wild-type (J; 100%, n=29) and tkk (K; 6%, n=36) embryos. Animal pole views. (L,M) Immunostaining with anti-b-tubulin antibody. Wild-type (L; 86%, n=14) and tkk (M; 81%, n=16) embryos at 20 mpf. Vegetal pole views. Scale bars: 200 mm in A-E,H-K; 140 mm in F,G; 20 mm in L,M. Maternal Syntabulin is sufficient to rescue ventralization of tkk mutant progeny To address whether the strong reduction of syntabulin expression causes the ventralization of tkk embryos, we attempted to rescue the mutant phenotype by injecting syntabulin mRNA into 1-cell stage embryos. Supplying syntabulin after egg activation was not sufficient to rescue ventralization in tkk mutant progeny (data not shown). The failure of these rescue attempts, and the localization of syntabulin transcripts at the vegetal pole of early embryos (Fig. 2C-E), suggest that Syntabulin functions in the zygote during early cleavage stages or earlier during oogenesis. With this in mind, we generated transgenic fish that express syntabulin in tkk oocytes (Fig. 3). We constructed the rescue plasmid in the Tol2 vector (Kawakami et al., 2004) to contain the syntabulin cDNA, the first intron and 3⬘ UTR, GFP, and the zona pellucida protein C (zpc; zpcx) promoter, which drives maternal expression (Onichtchouk et al., 2003) (Fig. 3A). The rescue plasmid and transposase RNA were injected into embryos obtained from crosses of relatively old tkk homozygotes, the offspring of which show only mild, or no, ventralized phenotypes (Nojima et al., 2004). These fish were raised to adulthood (see Fig. S6 in the supplementary material). Maternal expression of the transgene was observed in a subset of progeny from these tkk mutant females based on GFP visualization at the 1-cell stage, prior to activation of zygotic gene expression. In total, from three crosses 80.7% of GFP-negative (non-transgenic) tkk embryos were completely ventralized, and only 9.3% showed morphologically normal DV patterning (Fig. 3B) at 24 hpf. By contrast, in the GFP-positive (transgenic) class, only 43.7% of the embryos were completely ventralized, whereas 40.3% displayed normal DV patterning (Fig. 3B). dha expression was also restored in transgenic tkk embryos (Fig. 3C,D). These data indicate that maternal syntabulin rescues the tkk axis defects, and provide evidence that reduced syntabulin expression in oocytes causes ventralization of tkk progeny. Dorsal termination by a motor protein linker RESEARCH ARTICLE 5 syntabulin mRNA in zebrafish. The syntabulin gene is composed of five exons and four introns (Fig. 4F). We constructed a rescue plasmid that contains all the exons and introns, and inserted the 2A peptide sequence of porcine teschovirus-1 (PTV1) and Venus (a YFP variant) cDNA between the coding region and the 3⬘ UTR (Fig. 4G). Transgenic embryos were generated by co-injection with transposase RNA (see Fig. S6 in the supplementary material). Of Venus-negative (non-transgenic) tkk embryos, 78.3% showed complete ventralization and 13% showed normal DV patterning, whereas only 30.1% of Venus-positive (transgenic) tkk embryos displayed complete ventralization and 59.4% displayed normal DV patterning (Fig. 4H). This result indicates that this exon/introncontaining plasmid rescued the tkk phenotypes more efficiently than the plasmid containing the cDNA, first intron and 3⬘ UTR. This rescue was also more efficient than that by a plasmid containing the cDNA and 3⬘ UTR (data not shown). dha expression was also more efficiently restored by this plasmid, compared with the cDNA plus first intron and 3⬘ UTR construct (Fig. 4I,J). Furthermore, more syntabulin mRNA was localized to the vegetal pole in tkk embryos rescued by this plasmid (asterisk in Fig. 4K versus 4L as a control). These findings indicate that the exonintron structure is required for vegetal pole localization and proper function of syntabulin. Microtubule-dependent transport of Syntabulin Syntabulin is a linker protein that attaches cargo to kinesin I, and in neurons Syntabulin functions in cargo transport to the synaptic terminal along microtubules (Cai et al., 2005; Cai et al., 2007; Su et al., 2004) (see Fig. S3 in the supplementary material). We found that kif5b, which encodes the heavy chain of kinesin I, is expressed in zebrafish oocytes (see Fig. S7 in the supplementary material). When HA-tagged Kif5b and Myc-tagged Syntabulin were co-expressed in human embryonic kidney (HEK) 293T cells, Syntabulin coimmunoprecipitated with Kif5b (Fig. 5A). These data indicate that zebrafish Syntabulin can interact with the motor protein kinesin I that functions to transport cargo along microtubules. We next examined the localization of Syntabulin protein. We raised one polyclonal and two monoclonal antibodies against Syntabulin (see Fig. S8 in the supplementary material). All the antibodies detected Syntabulin at the vegetal pole in the wild type but not in tkk embryos from just after fertilization through 20 mpf (Fig. 5B-G and see Fig. S8C,D in the supplementary material). After 20 mpf, when microtubule array formation occurs at the vegetal pole (Jesuthasan and Stahle, 1997), Syntabulin redistributed to one side of the vegetal pole (Fig. 5H-J). This biased localization of Syntabulin was detected through 50 mpf (the 2-cell stage, Fig. 5J). Furthermore, Syntabulin remained at the vegetal pole in embryos DEVELOPMENT Fig. 2. syntabulin is responsible for the tkk mutant phenotype. (A) Linkage mapping. The zebrafish tkk locus was initially mapped near the SSLP marker z1543. Seven BAC clones were used to construct a physical map. Linkage analysis (numbers indicate recombinants per meiosis), sequence analysis of BAC clones and database searches revealed that there were 12 candidate genes in the chromosomal region. (B) Quantification of syntabulin (gene 6 in A) expression in wild-type and tkk embryos (expression in wild type taken as 100; *, P<0.01; the average ±s.d. is shown). zdhhc3 (zinc-finger, DHHC-type containing 3, gene 3) and e(y)2 (enhancer of yellow gene, gene 8) are located in the same chromosomal region as syntabulin and were used as controls. The expression of other genes in this chromosomal region is shown in Table S1 in the supplementary material. (C-F) Expression and localization of syntabulin RNA in an unfertilized wild-type egg (5 minutes after activation, C), in a 1-cell stage wild-type embryo (20 mpf, D), an 8-cell stage wild-type embryo (E), and in a 1-cell stage tkk embryo (F). Lateral views; A, animal pole; Vg, vegetal pole. (G) Molecular nature of the syntabulintkk4 allele. PCR detection of the insertion in wild-type and tkk embryos (n=3 each). Scale bars: 200 mm. 6 RESEARCH ARTICLE Development 137 (6) Fig. 3. Maternal expression of syntabulin rescues tkk mutants. (A) Structure of the rescue plasmid. Tol2, inverted repeats of Tol2 transposon; zpc, promoter of zone pellucida protein C; 3⬘ UTR, 3⬘ untranslated region; pAS, polyadenylation signal. (B) Rescue by syntabulin cDNA transgene. The numbers of transgenic (green) and non-transgenic (orange) zebrafish embryos showing each phenotype are tabulated, and the percentages are shown in the bar chart. (C,D) Expression of dha in tkk (D; 8%, n=25) and transgene-rescued (C, GFP+; 48%, n=33) embryos at the late blastula stage. (E,F) Localization of syntabulin at the 1-cell stage in a transgenerescued (E; 67%, n=27) versus control (F; 100%, n=23) embryo. Scale bars: 200 mm. syntabulin RNA is transported to the vegetal pole through the Buc-mediated Balbiani bodydependent pathway Some vegetal RNAs, including germline-specific gene products, are transported to the vegetal pole through the Balbiani body (Kloc et al., 2001; Kloc and Etkin, 1995; Kloc et al., 1996; Wilk et al., 2005), which requires maternal Buc function for its formation (Bontems et al., 2009; Marlow and Mullins, 2008). As syntabulin mRNA localizes to the vegetal pole of eggs and early embryos, we investigated when syntabulin achieves its vegetal pole localization and whether this involves the Buc-mediated Balbiani bodydependent pathway in oocytes. During oogenesis, syntabulin mRNA was detected in the Balbiani body of wild-type stage Ib oocytes (compare Fig. 6A with 6B,E), but was not localized in buc mutant oocytes (Fig. 6C,D,F). This was not due to reduced expression or degradation of syntabulin mRNA, as similar levels were detected by qRT-PCR in wild-type and buc mutant stage I-III oocytes (Fig. 6G). In unfertilized buc mutant eggs, syntabulin mRNA showed circumferential localization, indicating that the later localization of Fig. 4. Exon-intron structure of syntabulin is required for vegetal localization and proper dorsal determination. (A-E) The 3⬘ UTR is not sufficient for vegetal localization of syntabulin. (A) Structure of the transgene plasmid. (B-E) Localization of GFP mRNA in transgenic (B,D) or non-transgenic (C,E) tkk (D,E) or wild-type (B,C) zebrafish embryos at the 1-cell stage (20 mpf). (F,G) Genomic structure of syntabulin (F) and the rescue plasmid that contains the exon-intron structure of syntabulin. 2A-peptide, 2A peptide of PTV1. (H) Rescue by genomic DNA. The numbers (tabulated) and percentages (bar chart) of transgenic (green) and non-transgenic (orange) embryos showing each phenotype. (I,J) Expression of dha in tkk (J; 9%, n=32) and rescued (I; 66%, n=33) embryos at the late blastula stage. (K,L) Localization of syntabulin at the 1-cell stage in a rescued (K; 86%, n=35) versus control (L; 100%, n=31) embryo. Shown are animal pole views (I,J) and lateral views (A-E,K,L). Vegetal pole localization is marked by an asterisk (K). Scale bars: 200 mm. syntabulin to the vegetal pole depends on its proper localization in the Balbiani body of early oocytes (Fig. 6H-J). These data suggest that vegetal pole localization of syntabulin mRNA is mediated through the Buc-mediated Balbiani body-dependent pathway, which is essential for AV polarity in oocytes. DISCUSSION Buc-mediated Balbiani body-dependent transport of syntabulin RNA syntabulin mRNA localization to the vegetal pole of unfertilized eggs and early stage embryos is established during oogenesis (Fig. 2C-E and Fig. 6). In early stage wild-type oocytes, syntabulin transcripts localize to the Balbiani body (Fig. 6B,E). Balbiani body localization of syntabulin mRNA was disrupted in buc mutant oocytes (Fig. 6I,J), which lack the Balbiani body and show aberrant localization of vegetal RNAs during establishment of oocyte AV polarity (Bontems et al., 2009; Dosch et al., 2004; Marlow and Mullins, 2008). These data indicate that, after syntabulin RNA is transcribed, it is transported to the vegetal pole through the Balbiani DEVELOPMENT treated with nocodazole or colchicine, which are inhibitors of microtubule formation (Fig. 5M-Q), indicating that Syntabulin translocation is microtubule dependent. After 60 mpf, Syntabulin was not detected by whole-mount immunohistochemistry (Fig. 5K), nor in whole embryonic lysates by immunoprecipitation and immunoblotting (Fig. 5L), although syntabulin mRNA persisted at the vegetal pole through the 16-cell stage (90 mpf, Fig. 2E and see Fig. S4A in the supplementary material). These data indicate that the inability to detect Syntabulin after 60 mpf was most likely due to its degradation, rather than to protein diffusion or a decline in syntabulin mRNA abundance. Together, our findings reveal that the localization and stability of Syntabulin are tightly controlled after fertilization until the protein is no longer detected around the 2-cell stage. Fig. 5. Microtubule-dependent transportation of Syntabulin. (A) Interaction between Syntabulin and Kif5b. HEK293T cells were transfected with expression vectors of Myc-tagged Syntabulin and HAtagged Kif5b. IP, immunoprecipitation; IB, immunoblotting. (B-E) Detection of Syntabulin with anti-Syntabulin monoclonal antibodies (anti-Syn1, anti-Syn2) in wild-type and tkk zebrafish embryos at early zygote stage (10 mpf). Expression of Syntabulin at the animal pole (asterisks in D,E) is non-specific as it is detected in tkk embryos, which lack significant amounts of Syntabulin (see Fig. S8C in the supplementary material). (F-K) Time course of Syntabulin localization in the wild type. Immunostaining with anti-Syn1. (L) Immunoblotting of anti-Syntabulin (anti-Syn2) immunoprecipitates. (M-O) Localization of Syntabulin at 45 mpf in wild-type embryos treated with 1 mg/ml nocodazole (N) or 0.4 mg/ml colchicine (O) just after fertilization, versus untreated control (M). (P,Q) Quantification of Syntabulin localization at 45 mpf showing lopsided or vegetal pole localization, or undetectable expression in embryos treated with nocodazole (P) or colchicine (Q), versus control (P,Q). Shown are lateral views with animal pole to the top. Scale bars: 200 mm. body pathway during oogenesis (Fig. 7). The Balbiani bodydependent RNA transport system is shared by germline-specific RNAs (Kloc et al., 2001; Kloc and Etkin, 1995; Kloc et al., 1996; Kosaka et al., 2007; Wilk et al., 2005). Despite their common localization in early oocytes, important differences exist between syntabulin and germline-specific mRNA localization in late stage oocytes and in embryos. First, after the initial localization of germline-specific mRNAs to the Balbiani body during oogenesis, some of these transcripts, such as dazl (Maegawa et al., 1999; Kosaka et al., 2007), remain at the vegetal pole, whereas others, such RESEARCH ARTICLE 7 Fig. 6. Balbiani body-mediated localization of syntabulin at the vegetal pole. (A-D) Localization of syntabulin RNA in wild-type and bucky ball mutant (bucp106 and bucp43) stage Ib (STI/Ib) zebrafish oocytes. Staining by whole-mount in situ hybridization with sense (A, control) or antisense (B-D) riboprobes. In wild-type oocytes, syntabulin RNA localizes to the Balbiani body (A,B; 84%, n=104). In bucp106 (C; 97%, n=87 oocytes from three females) and bucp43 (D; 100%, n=36 from one female) mutant oocytes, syntabulin is not spatially restricted or localized. (E,F) Localization of syntabulin RNA. In wild-type oocytes (E), syntabulin localizes to the Balbiani body, whereas syntabulin is detected throughout the cytoplasm in bucp106 mutants (F; 100%, n=27 from one female). (G) Quantification of syntabulin expression in wildtype and buc oocytes. Although syntabulin is not localized in buc mutants, the transcripts are present in early and late stage oocytes. (H-J) Localization of syntabulin RNA in wild-type and buc activated eggs. In activated eggs from wild type, syntabulin shows polarized localization (H; 100% n=37 from two females). In activated eggs from bucp106 (I; 100%, n=44 from three females) and bucp43 (J; 100%, n=8 from one female) mutants, syntabulin is detected around the circumference. Arrows indicate localization of syntabulin at the vegetal pole in wild type and its circumferential expansion in buc eggs. nuc, nucleus; Bb, Balbiani body. Scale bars: 50 mm. as vasa (Knaut et al., 2000), redistribute around the circumference of the oocyte cortex or, such as nanos (Draper et al., 2007), are found throughout late stage oocytes. Despite their distinct positions in late stage oocytes, the germline-specific mRNAs are transported to the animal pole during egg activation, and subsequently localize to the distal ends of the blastomere cleavage furrows, where germ plasm is thought to be present during early cleavage stages (Hashimoto et al., 2004; Kosaka et al., 2007; Maegawa et al., 1999; Suzuki et al., 2000). By contrast, syntabulin mRNA remained at the vegetal pole until the 16-cell stage, and was not transported to the cleavage furrows or to the primordial germ cells (Fig. 2E and see Fig. S4 in the supplementary material). Second, whereas the 3⬘ UTR is sufficient for localization of germline-specific mRNAs to the vegetal pole (Kosaka et al., 2007), the 3⬘ UTR of syntabulin was not sufficient for its vegetal localization (Figs 3 and 4). Therefore, although both syntabulin and the germline-specific mRNAs rely on the Balbiani body for transport to the vegetal pole, they may utilize distinct mechanisms for later aspects of their localization. In Drosophila oocytes, posterior localization of oskar mRNA requires elements within its 3⬘ UTR and proper splicing, and occurs by a mechanism that is proposed to involve the exon-exon junction complex (EJC), which includes the nuclear shuttling proteins DEVELOPMENT Dorsal termination by a motor protein linker RESEARCH ARTICLE Fig. 7. The role of Syntabulin in dorsal determination in zebrafish. During oogenesis, syntabulin RNA is transported from the nucleus to the vegetal pole through the Buc-mediated Balbiani bodydependent pathway. During early embryogenesis, Syntabulin links the dorsal determinants (DDs) to Kif5B, the heavy chain of kinesin I. At 20 mpf, a microtubule array forms and Syntabulin transports the DDs to the plus end of microtubules. Around the 2-cell stage (60 mpf), Syntabulin is degraded and releases the DDs, which are eventually incorporated by dorsal blastomeres and activate zygotic gene expression cascades required for the formation of the dorsal organizer. Tsunagi (Y14) and Mago nashi (Hachet and Ephrussi, 2004). syntabulin mRNA produced from the transgene containing the exonintron structure and 3⬘ UTR was more enriched at the vegetal pole than RNA from the transgene containing the cDNA, first intron and 3⬘ UTR (Figs 3 and 4), suggesting that splicing of syntabulin RNA, and its Buc-mediated Balbiani body-dependent transport, also involve EJC components. syntabulin mRNA expressed from the transgene containing the full genomic structure of syntabulin, except the promoter and enhancer, was localized to the vegetal pole, but was also detected within the blastoderm (Fig. 4K). This is possibly due to the presence of the 2A peptide sequence and the Venus cDNA between the coding region and the 3⬘ UTR, which might affect the structure of nascent syntabulin mRNA and its transport. Alternatively, proper expression levels of syntabulin RNA might be required for its vegetal localization; for example, if components that localize or tether syntabulin to the vegetal pole are limiting. Future studies will reveal the precise molecular mechanisms that control the vegetal pole localization of syntabulin RNA. The link between AV oocyte polarity and DV axis formation The DDs initially localize to the vegetal pole of embryos. It is anticipated that AV oocyte polarity is linked to dorsal determination, although this possibility has not been formally tested. We show that syntabulin gene products, which are necessary for dorsal determination, localize to the vegetal pole in a Buc-mediated Balbiani body-dependent manner. Our findings provide evidence that links early AV polarity in oocytes to DV embryonic axis formation. Since Syntabulin functions as a cargo linker (Cai et al., 2005; Cai et al., 2007; Su et al., 2004), we hypothesize that Syntabulin localizes the DDs to the vegetal pole during oogenesis and/or in early stage zygotes (Fig. 7). Development 137 (6) When tkk embryos were rescued with the plasmid containing the cDNA, first intron and 3⬘ UTR of syntabulin, the transgene-derived RNA was not enriched at the vegetal pole (Fig. 3E), implying that vegetal pole localization of syntabulin RNA is not strictly required for dorsal determination. Alternatively, because syntabulin RNA was present throughout the embryo, it is possible that the amount of syntabulin RNA at the vegetal pole was sufficient for dorsal determination. Moreover, the rescue data suggest that additional factors contribute to localizing the DDs to the vegetal pole. Importantly, RNA from the plasmid containing the syntabulin exonintron structure was enriched at the vegetal pole and rescued the tkk phenotypes more efficiently (Fig. 4K). Therefore, vegetal localization of syntabulin RNA might ensure that Syntabulin protein is positioned to localize the DDs at the vegetal pole to facilitate their robust transport to the prospective dorsal side. Recently, a Balbiani body-localized ubiquitin ligase, Trim36, was identified that regulates microtubule polymerization or stability in the vegetal cortex of Xenopus (Cuykendall and Houston, 2009). Depletion of Trim36 leads to ventralization in Xenopus embryos (Cuykendall and Houston, 2009). Notably, like other Balbiani-localized transcripts, trim36 associates with the germ plasm in Xenopus embryos (Cuykendall and Houston, 2009). Our data identify syntabulin as a novel Balbiani bodylocalized mRNA. We find that unlike other Balbiani bodylocalized mRNAs, syntabulin is not a component of germ plasm in embryos, but instead mediates localization and transport of the DDs. Role of Syntabulin in dorsal determination Removal of the vegetal yolk or disruption of microtubule array formation during zygote or early cleavage stages perturbs the development of dorsal structures in zebrafish (Jesuthasan and Stahle, 1997; Mizuno et al., 1999; Ober and Schulte-Merker, 1999). These studies established the hypothesis that the DDs, initially localized to the vegetal pole, are subsequently transported to the prospective dorsal side along microtubules, where they are incorporated by dorsal blastomeres. We show that Syntabulin, an essential factor for dorsal determination in zebrafish, is initially localized to, and later translocates from, the vegetal pole (Fig. 5B-K). Syntabulin functions as a kinesin I motor protein linker that transports cargo from the soma to the presynaptic terminal of neuronal axons along microtubules towards their plus ends (Cai et al., 2005; Cai et al., 2007; Su et al., 2004). We demonstrate that zebrafish Syntabulin can interact with zebrafish Kif5b, a maternally expressed kinesin I heavy chain (Fig. 5A and see Fig. S7 in the supplementary material). Translocation of Syntabulin from the vegetal pole was microtubule dependent (Fig. 5B-K). Together, our results strongly suggest that Syntabulin links the DDs to kinesin I to mediate their initial vegetal pole localization and subsequent transport to the prospective dorsal side along the microtubule array that emanates from the vegetal pole (Fig. 7). Our results revealed that although maternal syntabulin RNA persisted through the 16-cell stage, Syntabulin protein was absent from around the 2-cell stage (Fig. 5K,L). It is unlikely that translation of Syntabulin is suppressed in this limited period. We favor the hypothesis that Syntabulin protein is degraded around the 2-cell stage. The rapid degradation of Syntabulin protein temporally coincides with stages when dorsalizing activity vacates the vegetal yolk (Mizuno et al., 1999; Ober and Schulte-Merker, 1999), indicating a possible mechanism whereby Syntabulin first positions the DDs and subsequently releases them from the vegetal microtubules following its own degradation (Fig. 7). DEVELOPMENT 8 How are the DDs transported to the dorsal blastomeres after their release from Syntabulin protein? Inhibition of microtubule formation at 8- or 16-cell stages leads to defective organizer formation (Jesuthasan and Stahle, 1997). Vegetal microtubules are only transiently detected in zygotes before the first cleavage, but are not detected at the 8- or 16-cell stage (Jesuthasan and Stahle, 1997). At these later stages, long microtubule arrays emanate from the marginal blastomeres (Jesuthasan and Stahle, 1997; Solnica-Krezel and Driever, 1994). Whether these microtubule arrays contribute to DD transport to the dorsal blastomeres requires further study. Our findings reveal two steps in DD transport from the vegetal pole to the dorsal blastomeres. Initially, the DDs are localized within the vegetal yolk. The first phase involves asymmetric translocation of the DDs via the vegetal microtubules, followed by their release from the vegetal portion of the yolk. Second, the DDs are transported to, and incorporated by, the prospective dorsal blastomeres. Syntabulin is essential for the first phase of DD transport (Fig. 7). Dorsal determinants In zebrafish, transcripts of the nodal-related gene ndr1 localize asymmetrically in a microtubule-dependent manner, which predicts the dorsal side of the embryos, and antisense morpholino-mediated knockdown of ndr1 affects the formation of dorsal structures (Gore et al., 2005). In contrast to syntabulin transcripts, which are localized to the vegetal pole of early oocytes and embryos, ndr1 mRNA is uniformly distributed throughout oocytes and localizes to the animal pole during egg activation before the first cleavage (Gore and Sampath, 2002). During this time in development, syntabulin mRNA and protein remain in the vegetal yolk (Fig. 2C,E and Fig. 6). Later, during early cleavage stages, ndr1 mRNA localizes asymmetrically within the blastomeres of the 2- to 4-cell stage embryo (Gore et al., 2005). At this time, maternal Syntabulin protein is no longer detected in the embryo (Fig. 5J,K). Moreover, asymmetric localization of ndr1 is b-catenin independent (Gore et al., 2005), and Nodal signaling is not required before the midblastula transition (Hagos and Dougan, 2007; Hagos et al., 2007), after b-catenin is detected in the nuclei of dorsal blastomeres (Fig. 1) (Dougan et al., 2003). Although it is possible that Syntabulin is involved in ndr1 mRNA transport, the reports discussed above and our spatial-temporal analysis of Syntabulin localization and function suggest that the mechanisms that mediate the translocation of ndr1 and the Wnt pathway-activating DDs are distinct. The notion that distinct mechanisms contribute to translocation of the DDs and ndr1 is compatible to a model whereby Syntabulin transports the DDs to activate b-catenin, which then induces essential zygotic regulators of axis formation, including ndr1 (Bennett et al., 2007; Dougan et al., 2003; Shimizu et al., 2000b). In Xenopus, maternal Wnt11 and Wnt5a function in dorsal determination (Cha et al., 2008; Tao et al., 2005). wnt11 mRNA localizes to the Balbiani body (Kloc and Etkin, 1995) and to the vegetal pole (Ku and Melton, 1993) of Xenopus oocytes. Wnt11 protein is enriched dorsally in cleavage-stage Xenopus embryos (Schroeder et al., 1999). Depletion of maternal wnt11 from late stage Xenopus oocytes leads to dorsal axis defects (Tao et al., 2005). In zebrafish, expression of wnt11/wnt11r and wnt5a/b genes is weak or below detection in 1-cell stage embryos, as compared with gastrula and/or pharyngula stages (see Fig. S9 in the supplementary material) when these Wnts function in cell migration (Heisenberg et al., 2000; Matsui et al., 2005; Rauch et al., 1997). Maternal zygotic wnt11 (silberblick) mutant embryos do not exhibit disrupted DV axis formation (Heisenberg et al., RESEARCH ARTICLE 9 2000). Injection of wnt5a/b and/or wnt11/11r RNA just after fertilization impairs gastrulation movements (data not shown), but neither affected dha expression in wild-type embryos nor rescued dha expression in tkk embryos (see Fig. S10 in the supplementary material), suggesting negligible contributions of these Wnts to Syntabulin-mediated dorsal determination. Furthermore, overexpression of the Wnt signaling components Dishevelled 3 (Dvl3), Glycogen synthase kinase (Gsk) binding protein (Gbp), dominant-negative Axin1 or dominant-negative Gsk3b rescued and/or induced hyper-dorsalization in tkk embryos; by contrast, Wnt8, Wnt3a or Frizzled 8b did not efficiently dorsalize tkk embryos (Nojima et al., 2004). These data suggest that Syntabulin functions downstream of Wnt receptors and upstream of bcatenin, in agreement with the hypothesis that Syntabulin does not transport Wnt RNAs or proteins. In Xenopus, Dishevelled (Dvl) and Gbp undergo microtubule- and/or motor protein-dependent transport and contribute to dorsal axis determination (Miller et al., 1999; Weaver et al., 2003; Yost et al., 1998). In early zebrafish embryos, biased localization of Dvl2, Dvl3 and Gbp was not detected (data not shown). Therefore, zebrafish and Xenopus may utilize different DDs, or there are DDs that are shared among vertebrate species that remain to be discovered. In either case, the answer to this question requires the isolation and identification of the DDs. As a linker protein that attaches cargo, presumably including the DDs, to kinesin I, Syntabulin might facilitate identification of the elusive DDs. In summary, the regulation of dorsal determination via microtubule dynamics and association with motor and adaptor proteins is shared among zebrafish and amphibians. Our findings provide a missing component of the microtubule-dependent DD transport machinery. Moreover, our finding that syntabulin, an essential regulator of dorsal axis formation, relies on early AV oocyte polarity for its localization provides new insight into the link between the first embryonic axis and dorsal determination in zebrafish. Acknowledgements We thank K. Kawakami for the Tol2 transposon system; M. Tsang, M. Tada, D. Onichtchouk, T. Matsui and J. C. Belmonte for plasmids; M. Mullins for comments on the manuscript; K. Bando, S. Fujii, A. Katsuyama, S. Onishi for technical assistance; and the members of the Hibi laboratory for helpful discussions. This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Technology, Japan (21370103 to M.H.). Competing interests statement The authors declare no competing financial interests. Supplementary material Supplementary material for this article is available at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.046425/-/DC1 References Bellipanni, G., Varga, M., Maegawa, S., Imai, Y., Kelly, C., Myers, A. P., Chu, F., Talbot, W. S. and Weinberg, E. S. (2006). Essential and opposing roles of zebrafish beta-catenins in the formation of dorsal axial structures and neurectoderm. Development 133, 1299-1309. Bennett, J. T., Stickney, H. L., Choi, W. Y., Ciruna, B., Talbot, W. S. and Schier, A. F. (2007). Maternal nodal and zebrafish embryogenesis. Nature 450, E1-E2; discussion E2-E4. Bontems, F., Stein, A., Marlow, F., Lyautey, J., Gupta, T., Mullins, M. C. and Dosch, R. (2009). Bucky ball organizes germ plasm assembly in zebrafish. Curr. Biol. 19, 414-422. Cai, Q., Gerwin, C. and Sheng, Z. H. (2005). Syntabulin-mediated anterograde transport of mitochondria along neuronal processes. J. Cell Biol. 170, 959969. Cai, Q., Pan, P. Y. and Sheng, Z. H. (2007). Syntabulin-kinesin-1 family member 5B-mediated axonal transport contributes to activity-dependent presynaptic assembly. J. Neurosci. 27, 7284-7296. DEVELOPMENT Dorsal termination by a motor protein linker RESEARCH ARTICLE Cha, S. W., Tadjuidje, E., Tao, Q., Wylie, C. and Heasman, J. (2008). Wnt5a and Wnt11 interact in a maternal Dkk1-regulated fashion to activate both canonical and non-canonical signaling in Xenopus axis formation. Development 135, 3719-3729. Cuykendall, T. N. and Houston, D. W. (2009). Vegetally localized Xenopus trim36 regulates cortical rotation and dorsal axis formation. Development 136, 3057-3065. De Robertis, E. M. (2006). Spemann’s organizer and self-regulation in amphibian embryos. Nat. Rev. Mol. Cell Biol. 7, 296-302. De Robertis, E. M., Larrain, J., Oelgeschlager, M. and Wessely, O. (2000). The establishment of Spemann’s organizer and patterning of the vertebrate embryo. Nat. Rev. Genet. 1, 171-181. Dosch, R., Wagner, D. S., Mintzer, K. A., Runke, G., Wiemelt, A. P. and Mullins, M. C. (2004). Maternal control of vertebrate development before the midblastula transition: mutants from the zebrafish I. Dev. Cell 6, 771-780. Dougan, S. T., Warga, R. M., Kane, D. A., Schier, A. F. and Talbot, W. S. (2003). The role of the zebrafish nodal-related genes squint and cyclops in patterning of mesendoderm. Development 130, 1837-1851. Draper, B. W., McCallum, C. M. and Moens, C. B. (2007). nanos1 is required to maintain oocyte production in adult zebrafish. Dev. Biol. 305, 589-598. Elinson, R. P. and Rowning, B. (1988). A transient array of parallel microtubules in frog eggs: potential tracks for a cytoplasmic rotation that specifies the dorsoventral axis. Dev. Biol. 128, 185-197. Gerhart, J., Danilchik, M., Doniach, T., Roberts, S., Rowning, B. and Stewart, R. (1989). Cortical rotation of the Xenopus egg: consequences for the anteroposterior pattern of embryonic dorsal development. Development 107, 37-51. Gore, A. V. and Sampath, K. (2002). Localization of transcripts of the zebrafish morphogen Squint is dependent on egg activation and the microtubule cytoskeleton. Mech. Dev. 112, 153-156. Gore, A. V., Maegawa, S., Cheong, A., Gilligan, P. C., Weinberg, E. S. and Sampath, K. (2005). The zebrafish dorsal axis is apparent at the four-cell stage. Nature 438, 1030-1035. Guraya, S. S. (1979). Recent advances in the morphology, cytochemistry, and function of Balbiani’s vitelline body in animal oocytes. Int. Rev. Cytol. 59, 249321. Hachet, O. and Ephrussi, A. (2004). Splicing of oskar RNA in the nucleus is coupled to its cytoplasmic localization. Nature 428, 959-963. Hagos, E. G. and Dougan, S. T. (2007). Time-dependent patterning of the mesoderm and endoderm by Nodal signals in zebrafish. BMC Dev. Biol. 7, 22. Hagos, E. G., Fan, X. and Dougan, S. T. (2007). The role of maternal Activin-like signals in zebrafish embryos. Dev. Biol. 309, 245-258. Hashimoto, Y., Maegawa, S., Nagai, T., Yamaha, E., Suzuki, H., Yasuda, K. and Inoue, K. (2004). Localized maternal factors are required for zebrafish germ cell formation. Dev. Biol. 268, 152-161. Heisenberg, C. P., Tada, M., Rauch, G. J., Saude, L., Concha, M. L., Geisler, R., Stemple, D. L., Smith, J. C. and Wilson, S. W. (2000). Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature 405, 76-81. Hibi, M., Hirano, T. and Dawid, I. B. (2002). Organizer formation and function. Results Probl. Cell Differ. 40, 48-4871. Houliston, E. and Elinson, R. P. (1991). Evidence for the involvement of microtubules, ER, and kinesin in the cortical rotation of fertilized frog eggs. J. Cell Biol. 114, 1017-1028. Jesuthasan, S. and Stahle, U. (1997). Dynamic microtubules and specification of the zebrafish embryonic axis. Curr. Biol. 7, 31-42. Kawakami, K., Takeda, H., Kawakami, N., Kobayashi, M., Matsuda, N. and Mishina, M. (2004). A transposon-mediated gene trap approach identifies developmentally regulated genes in zebrafish. Dev. Cell 7, 133-144. Kelly, C., Chin, A. J., Leatherman, J. L., Kozlowski, D. J. and Weinberg, E. S. (2000). Maternally controlled (beta)-catenin-mediated signaling is required for organizer formation in the zebrafish. Development 127, 3899-3911. Kloc, M. and Etkin, L. D. (1995). Two distinct pathways for the localization of RNAs at the vegetal cortex in Xenopus oocytes. Development 121, 287-297. Kloc, M., Larabell, C. and Etkin, L. D. (1996). Elaboration of the messenger transport organizer pathway for localization of RNA to the vegetal cortex of Xenopus oocytes. Dev. Biol. 180, 119-130. Kloc, M., Bilinski, S., Chan, A. P., Allen, L. H., Zearfoss, N. R. and Etkin, L. D. (2001). RNA localization and germ cell determination in Xenopus. Int. Rev. Cytol. 203, 63-91. Kloc, M., Bilinski, S. and Etkin, L. D. (2004). The Balbiani body and germ cell determinants: 150 years later. Curr. Top. Dev. Biol. 59, 1-36. Knapik, E. W., Goodman, A., Atkinson, O. S., Roberts, C. T., Shiozawa, M., Sim, C. U., Weksler-Zangen, S., Trolliet, M. R., Futrell, C., Innes, B. A. et al. (1996). A reference cross DNA panel for zebrafish (Danio rerio) anchored with simple sequence length polymorphisms. Development 123, 451-460. Knaut, H., Pelegri, F., Bohmann, K., Schwarz, H. and Nusslein-Volhard, C. (2000). Zebrafish vasa RNA but not its protein is a component of the germ plasm and segregates asymmetrically before germline specification. J. Cell Biol. 149, 875-888. Development 137 (6) Kosaka, K., Kawakami, K., Sakamoto, H. and Inoue, K. (2007). Spatiotemporal localization of germ plasm RNAs during zebrafish oogenesis. Mech. Dev. 124, 279-289. Ku, M. and Melton, D. A. (1993). Xwnt-11: a maternally expressed Xenopus wnt gene. Development 119, 1161-1173. Leung, T., Soll, I., Arnold, S. J., Kemler, R. and Driever, W. (2003). Direct binding of Lef1 to sites in the boz promoter may mediate pre-midblastulatransition activation of boz expression. Dev. Dyn. 228, 424-432. Maegawa, S., Yasuda, K. and Inoue, K. (1999). Maternal mRNA localization of zebrafish DAZ-like gene. Mech. Dev. 81, 223-226. Marlow, F. L. and Mullins, M. C. (2008). Bucky ball functions in Balbiani body assembly and animal-vegetal polarity in the oocyte and follicle cell layer in zebrafish. Dev. Biol. 321, 40-50. Matsui, T., Raya, A., Kawakami, Y., Callol-Massot, C., Capdevila, J., Rodriguez-Esteban, C. and Izpisua Belmonte, J. C. (2005). Noncanonical Wnt signaling regulates midline convergence of organ primordia during zebrafish development. Genes Dev. 19, 164-175. Mei, W., Lee, K. W., Marlow, F. L., Miller, A. L. and Mullins, M. C. (2009). hnRNP I is required to generate the Ca2+ signal that causes egg activation in zebrafish. Development 136, 3007-3017. Miller, J. R., Rowning, B. A., Larabell, C. A., Yang-Snyder, J. A., Bates, R. L. and Moon, R. T. (1999). Establishment of the dorsal-ventral axis in Xenopus embryos coincides with the dorsal enrichment of dishevelled that is dependent on cortical rotation. J. Cell Biol. 146, 427-437. Mizuno, T., Yamaha, E., Kuroiwa, A. and Takeda, H. (1999). Removal of vegetal yolk causes dorsal deficiencies and impairs dorsal-inducing ability of the yolk cell in zebrafish. Mech. Dev. 81, 51-63. Nagai, T., Ibata, K., Park, E. S., Kubota, M., Mikoshiba, K. and Miyawaki, A. (2002). A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat. Biotechnol. 20, 87-90. Nojima, H., Shimizu, T., Kim, C. H., Yabe, T., Bae, Y. K., Muraoka, O., Hirata, T., Chitnis, A., Hirano, T. and Hibi, M. (2004). Genetic evidence for involvement of maternally derived Wnt canonical signaling in dorsal determination in zebrafish. Mech. Dev. 121, 371-386. Ober, E. A. and Schulte-Merker, S. (1999). Signals from the yolk cell induce mesoderm, neuroectoderm, the trunk organizer, and the notochord in zebrafish. Dev. Biol. 215, 167-181. Onichtchouk, D., Aduroja, K., Belting, H. G., Gnugge, L. and Driever, W. (2003). Transgene driving GFP expression from the promoter of the zona pellucida gene zpc is expressed in oocytes and provides an early marker for gonad differentiation in zebrafish. Dev. Dyn. 228, 393-404. Provost, E., Rhee, J. and Leach, S. D. (2007). Viral 2A peptides allow expression of multiple proteins from a single ORF in transgenic zebrafish embryos. Genesis 45, 625-629. Rauch, G. J., Hammerschmidt, M., Blader, P., Schauerte, H. E., Strahle, U., Ingham, P. W., McMahon, A. P. and Haffter, P. (1997). Wnt5 is required for tail formation in the zebrafish embryo. Cold Spring Harb. Symp. Quant. Biol. 62, 227-234. Ryu, S. L., Fujii, R., Yamanaka, Y., Shimizu, T., Yabe, T., Hirata, T., Hibi, M. and Hirano, T. (2001). Regulation of dharma/bozozok by the Wnt pathway. Dev. Biol. 231, 397-409. Schier, A. F. and Talbot, W. S. (2005). Molecular genetics of axis formation in zebrafish. Annu. Rev. Genet. 39, 561-613. Schneider, S., Steinbeisser, H., Warga, R. M. and Hausen, P. (1996). Betacatenin translocation into nuclei demarcates the dorsalizing centers in frog and fish embryos. Mech. Dev. 57, 191-198. Schroeder, K. E., Condic, M. L., Eisenberg, L. M. and Yost, H. J. (1999). Spatially regulated translation in embryos: asymmetric expression of maternal Wnt-11 along the dorsal-ventral axis in Xenopus. Dev. Biol. 214, 288-297. Schroeder, M. M. and Gard, D. L. (1992). Organization and regulation of cortical microtubules during the first cell cycle of Xenopus eggs. Development 114, 699709. Shimizu, T., Yamanaka, Y., Ryu, S., Hashimoto, H., Yabe, T., Hirata, T., Bae, Y., Hibi, M. and Hirano, T. (2000a). Cooperative roles of Bozozok/Dharma and nodal-related proteins in the formation of the dorsal organizer in zebrafish. Mech. Dev. 91, 293-303. Shimizu, T., Yamanaka, Y., Ryu, S., Hashimoto, H., Yabe, T., Hirata, T., Bae, Y., Hibi, M. and Hirano, T. (2000b). Cooperative roles of Bozozok/Dharma and nodal-related proteins in the formation of the dorsal organizer in zebrafish. Mech. Dev. 91, 293-303. Shimizu, T., Yabe, T., Muraoka, O., Yonemura, S., Aramaki, S., Hatta, K., Bae, Y. K., Nojima, H. and Hibi, M. (2005). E-cadherin is required for gastrulation cell movements in zebrafish. Mech. Dev. 122, 747-763. Sirotkin, H. I., Dougan, S. T., Schier, A. F. and Talbot, W. S. (2000). bozozok and squint act in parallel to specify dorsal mesoderm and anterior neuroectoderm in zebrafish. Development 127, 2583-2592. Solnica-Krezel, L. and Driever, W. (1994). Microtubule arrays of the zebrafish yolk cell: organization and function during epiboly. Development 120, 24432455. DEVELOPMENT 10 Su, Q., Cai, Q., Gerwin, C., Smith, C. L. and Sheng, Z. H. (2004). Syntabulin is a microtubule-associated protein implicated in syntaxin transport in neurons. Nat. Cell Biol. 6, 941-953. Suzuki, H., Maegawa, S., Nishibu, T., Sugiyama, T., Yasuda, K. and Inoue, K. (2000). Vegetal localization of the maternal mRNA encoding an EDEN-BP/Brunolike protein in zebrafish. Mech. Dev. 93, 205-209. Tao, Q., Yokota, C., Puck, H., Kofron, M., Birsoy, B., Yan, D., Asashima, M., Wylie, C. C., Lin, X. and Heasman, J. (2005). Maternal wnt11 activates the canonical wnt signaling pathway required for axis formation in Xenopus embryos. Cell 120, 857-871. Thisse, C. and Thisse, B. (1998). High resolution whole-mount in situ hybridization. In Zebrafish Science Monitor, vol. 5, issue 1. Eugene, OR: University of Oregon Press (http://zfin.org/zf_info/monitor/vol5.1/vol5.1.html). Tsang, M., Maegawa, S., Kiang, A., Habas, R., Weinberg, E. and Dawid, I. B. (2004). A role for MKP3 in axial patterning of the zebrafish embryo. Development 131, 2769-2779. Weaver, C., Farr, G. H., 3rd, Pan, W., Rowning, B. A., Wang, J., Mao, J., Wu, D., Li, L., Larabell, C. A. and Kimelman, D. (2003). GBP binds kinesin light RESEARCH ARTICLE 11 chain and translocates during cortical rotation in Xenopus eggs. Development 130, 5425-5436. Westerfield, M. (1995). The Zebrafish Book: a Guide for the Laboratory Use of Zebrafish. Eugene, OR: University of Oregon Press. Wilk, K., Bilinski, S., Dougherty, M. T. and Kloc, M. (2005). Delivery of germinal granules and localized RNAs via the messenger transport organizer pathway to the vegetal cortex of Xenopus oocytes occurs through directional expansion of the mitochondrial cloud. Int. J. Dev. Biol. 49, 17-21. Yamanaka, Y., Mizuno, T., Sasai, Y., Kishi, M., Takeda, H., Kim, C. H., Hibi, M. and Hirano, T. (1998). A novel homeobox gene, dharma, can induce the organizer in a non-cell-autonomous manner. Genes Dev. 12, 2345-2353. Yonemura, S., Matsui, T. and Tsukita, S. (2002). Rho-dependent and independent activation mechanisms of ezrin/radixin/moesin proteins: an essential role for polyphosphoinositides in vivo. J. Cell Sci. 115, 2569-2580. Yost, C., Farr, G. H., 3rd, Pierce, S. B., Ferkey, D. M., Chen, M. M. and Kimelman, D. (1998). GBP, an inhibitor of GSK-3, is implicated in Xenopus development and oncogenesis. Cell 93, 1031-1041. DEVELOPMENT Dorsal termination by a motor protein linker