* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download C. jejuni
Fatty acid metabolism wikipedia , lookup
Pharmacometabolomics wikipedia , lookup
Ribosomally synthesized and post-translationally modified peptides wikipedia , lookup
Citric acid cycle wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Peptide synthesis wikipedia , lookup
Signal transduction wikipedia , lookup
Multilocus sequence typing wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Metabolic network modelling wikipedia , lookup
Point mutation wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Protein structure prediction wikipedia , lookup
Genetic code wikipedia , lookup
Proteolysis wikipedia , lookup
Biochemistry wikipedia , lookup
Glutathione wikipedia , lookup
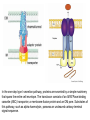
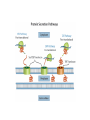
Metabolic Diversity in Campylobacter jejuni Enhances Specific Tissue Colonization Dirk Hofreuter,1 Veronica Novik,1 and Jorge E. Gala´ n1,* 1Section of Microbial Pathogenesis, Boyer Center for Molecular Medicine, Yale University School of Medicine, 295 Congress Avenue, New Haven, CT 06536, USA *Correspondence: [email protected] DOI 10.1016/j.chom.2008.10.002 Metabolic Diversity in Campylobacter jejuni Enhances Specific Tissue Colonization 30.11.2009 Campylobacter jejuni • a representative of the group of ε-proteobacteria • curved, rod-shaped, non-spore microaerophilic bacteria • one of the most common causes of human gastroenteriris in the world, leading cause of foodborne diarrhea in industrialized nations Campylobacter jejuni 30.11.2009 forming, Gram-negative, Infectious diseases - Bacterial diseases: Proteobacterial Infectious Diseases – Bacterial diseases: Proteobacterial 30.11.2009 Campylobacter jejuni • • • • • much of the genetic diversity among different isolates of C. jejuni is found in putative virulence factors mechanisms of C. jejuni pathogenesis that lead to such a variety of clinical presentations are very poorly understood cannot utilize sugars as a carbon source because it lacks the glycolytic enzyme phosphofructokinase is thought to depend mainly on the availability of free amino and keto acids from the host or from the intestinal microbial flora serine, aspartate, glutamate, and proline are preferentially used as nutritional substrates in vitro Campylobacter jejuni 30.11.2009 Campylobacter jejuni 81-176 Exhibits Expanded Ability to Utilize Amino Acids • C. jejuni 81-176 was able to grow in the presence of aspartate, glutamate, proline, and serine • C. jejuni 81-176 was growing in the presence of glutamine and asparagine, which did not sustain the growth of the reference strain NCTC 11168 Conclusion: C. jejuni 81-176 exhibits additional metabolic traits, which may enhance its virulence properties. Campylobacter jejuni 81-176 Exhibits Expanded Ability to Utilize Amino Acids 30.11.2009 NCTC 11168 γ-glutamyltranspeptidase (GGT) • • • • present in the cell membranes of many tissues involved in the transfer of amino acids across the cellular membrane catalyzes the degradation of glutathione to glutamate and a cysteinylglycine dipeptide through a transpeptidation reaction that transfers the g-glutamyl moiety to amino acids and peptides can hydrolyze glutathione and glutamine, releasing free glutamate. (5-L-glutamyl)-peptide + an amino acid peptide + 5-L-glutamyl amino acid A C. jejuni 81-176 gene (cju06), which is absent from the NCTC 11168 strain, encodes a secreted GGT. GGT is produced as a 60 kDa proenzyme that is processed upon periplasmic secretion into an active enzyme consisting of 40 kDa and 20 kDa subunits (GGT) FLAG epitope-tagged γ glutamyltranspeptidase Expression, localization, and processing of C. jejuni 81-176 γ glutamyltranspeptidase. (GGTDSP) Mutant derivative lacking its putative sec-dependent secretion signal Whole cell (WC) Periplasmic fractions (PP) Note that in the absence of the secretion signal, GGT is not efficiently processed into the 40 and 20 kDa subunits. γ-glutamyltranspeptidase 30.11.2009 A GGT Confers on C. jejuni 81-176 the Ability to Utilize Glutamine • Hypothesis the hydrolytic activity of GGT may lead to the deamination of glutamine, and hence allow its utilization by C. jejuni 81-176 via glutamate, the end product of this reaction. The isogenic ggt::kan mutant of C. jejuni 81-176 was unable to utilize glutamine for growth in DMEM. Ability of C. jejuni 81-176 and mutant derivatives to utilize glutamine, glutathione, and compounds derived from the activity of GGT C. jejuni 81-176 Ability to Utilize Glutamine 30.11.2009 G-Glutamyl Cycle A GGT Confers on C. jejuni 81-176 the Ability to Utilize Glutathione • • • does not encode homologs of the glutathione biosynthesis proteins glutathione is readily available in mammalian tissues glutathione has been shown to serve as a potential amino acid source for E. coli and Neisseria meningitidis in vitro Hypothesis GGT may allow C. jejuni to utilize glutathione as an amino acid source • wild-type C. jejuni 81-176 showed enhanced growth with glutathione in a concentration-dependent manner • addition of glutathione did not enhance the growth of ggt::kan mutant - GGT is required for glutathione utilization • supplementation with the dipeptide cysteinylglycine did not enhance the growth of C. jejuni 81-176 • addition of glutamate or g-glutamylcysteine enhanced the growth of C. jejuni 81-176 to levels equivalent to those resulting from the addition of glutathione. • g-glutamylcysteine did not enhance the growth of the ggt::kan mutant, although the growth of this strain was enhanced by the addition of glutamate, which does not require GGT for its utilization A GGT Confers on C. jejuni 81-176 the Ability to Utilize Glutamine and Glutathione • • • • To investigate the possibility that the growth-enhancing effect of glutathione and glutamine was due to the generation of glutamate after their GGT-mediated hydrolysis, we investigated the consequence of a mutation in peb1A Peb1A is an aspartate/glutamate-binding protein of an ABC transporter Disruption of peb1A effectively abrogated the ability of C. jejuni to utilize glutathione, glutamine, and the dipeptide g-glutamylcysteine Disruption of peb1A did not affect the GGT activity of C. jejuni Conclusion GGT allows the utilization of glutathione, glutamine, or the dipeptide g-glutamylcysteine by generating glutamate, which is then taken up by the Peb1Adependent transporter and used as a carbon source. Type I secretion: ABC- Transporter ATP-binding cassette transporters (ABC-transporter) are members of a protein superfamily that is one of the largest and most ancient families with representatives in all extant phyla from prokaryotes to humans. ABC transporters are transmembrane proteins that utilize the energy of ATP hydrolysis to carry out certain biological processes including translocation of various substrates across membranes and non-transportrelated processes such as translation of RNA and DNA repair. They transport a wide variety of substrates across extra- and intracellular membranes, including metabolic products, lipids and sterols, and drugs. Proteins are classified as ABC transporters based on the sequence and organization of their ATP-binding cassette (ABC) domain(s). In the one-step type I secretion pathway, proteins are secreted by a simple machinery that spans the entire cell envelope. The translocon consists of an IM ATPase-binding cassette (ABC) transporter, a membrane-fusion protein and an OM pore. Substrates of this pathway, such as alpha-haemolysin, possess an uncleaved carboxy-terminal signal sequence. A GGT Confers on C. jejuni 81-176 the Ability to Utilize Glutamine and Glutathione • C. jejuni is able to utilize glutamine and glutathione in a ggt-dependent manner, and extends previous biochemical observations in H. pylori indicating that this bacterium can take up glutamate released from glutathione or glutamine by its GGT activity. • The data also reveal significant variability in glutathione metabolism among different bacteria. Glutathione + H2O <=> Cys-Gly + L-Glutamate A GGT Confers on C. jejuni 81-176 the Ability to Utilize Glutamine and Glutathione 30.11.2009 Type II- secretion: Sec- dependent secretion A Secreted Asparaginase Confers on C. jejuni 81176 the Ability to Utilize Asparagine Hypothesis: • the utilization of asparagine by C. jejuni 81-176 would require either a specific transporter for this amino acid, or a periplasmic asparaginase capable of converting asparagine to aspartate • • • No homolog of an asparagine transporter was detected in any of the sequenced strains of C. jejuni Highly related, the sequences exhibit intriguing differences. While the amino terminus of the C. jejuni 81-176 AnsB has a canonical sec-dependent secretion signal sequence, such a signal sequence is absent from the AnsB homolog of C. jejuni NCTC11168 . Comparison of the nucleotide sequence of the 50 region of ansB in these strains revealed a striking sequence difference. In C. jejuni 81-176, this region shows the presence of an additional 40 nucleotides adding a secretion signal to an otherwise cytoplasmic asparaginase, resulting in a secreted form of this enzyme that allows the utilization of asparagine by C. jejuni 81176. A Secreted Asparaginase Confers on C. jejuni 81-176 the Ability to Utilize Asparagine 30.11.2009 A Secreted Asparaginase Confers on C. jejuni 81176 the Ability to Utilize Asparagine • • Introduction of a loss-of-function mutation in the ansB homolog of C. jejuni 81-176, or exchanging the ansB allele with a homolog lacking the putative sec-dependent secretion signal, abolished the ability of this strain to utilize asparagine. A Secreted Asparaginase Confers on C. jejuni 81-176 the Ability to Utilize Asparagine 30.11.2009 The introduction of the C. jejuni 81-176 ansB allele into the C. jejuni NCTC 11168 strain allowed this strain to utilize asparagine •AnsB containing the putative secretion signal was detected in the periplasmic fraction of C. jejuni 81-176, •while a mutant derivative expressing an allele lacking the putative secretion signal was detected only in the cytoplasmic fraction A Secreted Asparaginase Confers on C. jejuni 81176 the Ability to Utilize Asparagine • The addition of a secretion signal to an otherwise cytoplasmic asparaginase conferred to C. jejuni 81-176 the ability to deaminate asparagine to aspartate in the periplasm, thus allowing the utilization of asparagine as a nutritional source. • The presence of a putative alternative start site downstream from the first initiation codon in the 81-176 ansB sequence, could potentially generate a cytoplasmic form of this enzyme, which could presumably ensure the production of both secreted and cytoplasmic forms of this enzyme. • The cytoplasmic form of AnsB could be involved in metabolizing asparagine that could be generated by the cytoplasmic degradation of small peptides imported by specific transporters. • Introduction of an insertion mutation in the ggt locus of the C. jejuni strains abolished their ability to utilize glutamine. Conclusion the ggt-mediated metabolic expansion is a common occurrence in clinical isolates of C. jejuni A Secreted Asparaginase Confers on C. jejuni 81-176 the Ability to Utilize Asparagine 30.11.2009 Clinical Isolates of C. jejuni Exhibit Significant Metabolic Diversity • Was examined the predicted amino acid sequence of AnsB encoded in the C. jejuni strains whose nucleotide genome sequences are available. • The asparaginases of C. jejuni several isolates showed no predicted N-terminal secretion signal and therefore are presumably cytoplasmic. In contrast, it was found sec-dependent secretion signals in AnsB from C. jejuni other isolates. To expand the analysis of asparaginases to other C. jejuni strains, the nucleotide sequence of ansB in several clinical isolates was determined . In 5 out of 15 isolates, asparaginases containing a sec-dependent signal sequence were found. In all of these cases, the nucleotide sequence of the 50 region of ansB was identical to that of C. jejuni 81-176, suggesting a common origin for the DNA segment encoding the secretion signal. • • • Clinical Isolates of C. jejuni Exhibit Significant Metabolic Diversity •The ability to utilize asparagine for clinical isolates was tested. • It was found a strict correlation between the presence of a secdependent secretion signal in the predicted sequences of AnsB and their ability to utilize this amino acid. • In addition to the difference in the ability of C. jejuni strains to metabolize asparagine and glutamine, 1 of the 13 C. jejuni isolates tested were unable to grow with serine. • C. jejuni serine metabolism was shown to be important for the effective colonization of chicken. • Of all the amino acids tested, proline was the only one that was able to support the growth of all tested strains. Conclusion unexpected diversity in basic metabolic traits of different clinical isolates of C. jejuni, which may affect their pathogenic potential. C. jejuni 81-176 Exhibits Differential Nutritional Requirements for the Efficient Colonization of Different Tissues • The potential role of GGT in the ability of C. jejuni to colonize the liver of myd88-/-mice was investigated in competition experiments. • C. jejuni ggt mutant strain colonized the liver of infected animals in a manner indistinguishable from wild-type, suggesting that glutamine and/or glutathione may not be essential nutrients for growth in the liver. Uunique nutritional requirements for C. jejuni in different tissues, since glutathione and/or glutamine are important nutritional sources in the intestinal tract but appear to be dispensable for its growth in the liver. • C. jejuni 81-176 Exhibits Differential Nutritional Requirements for the Efficient Colonization of Different Tissues 30.11.2009 C. jejuni 81-176 Exhibits Differential Nutritional Requirements for the Efficient Colonization of Different Tissues • • • • It was tested whether asparagine may also be an important nutrient during infection by comparing the ability of wild-type C. jejuni 81-176 and the isogenic ansB::kan mutant to colonize mice in mixed infection experiments. Five weeks after oral administration of equal numbers both strains were recovered in similar numbers from intestinal tissues of colonized animals. ansB::kan mutant, as well as a mutant expressing an asparaginase without its secretion signal peptide, were significantly defective in their ability to colonize the liver of infected animals when administered systemically. Such defect was fully restored by introduction of the wild-type allele, but not by introduction of an allele lacking the secretion signal. C. jejuni 81-176 Exhibits Differential Nutritional Requirements for the Efficient Colonization of Different Tissues Conclusions • In the liver but not in the intestine, asparagine, presumably through its metabolization into asparatate, is an important nutrient for C. jejuni. • The reduced but detectable ability of the ansB::kan mutant of C. jejuni to colonize the liver suggests that nutrients other than asparagine may be also available at this site to support limited replication of this C. jejuni strain. • Together with the observations made with the ggt mutant, these results show a remarkable specificity in C. jejuni’s nutritional requirements to efficiently colonize different tissues: glutathione or glutamine and the derived glutamate in the intestine and asparagine or aspartate in the liver. C. jejuni 81-176 Exhibits Differential Nutritional Requirements for the Efficient Colonization of Different Tissues 30.11.2009 Conclusions • C. jejuni is capable of colonizing a diverse array of warm-blooded animals, and persists in various environmental habitats exhibits significant diversity in its ability to utilize basic nutrients. • The metabolic diversity translates in different capacity to colonize different tissues. The acquisition of specific metabolic pathways has enhanced the ability of a pathogen to colonize different tissues. • It is possible that the expanded ability of this C. jejuni strain to utilize amino acids may contribute to its apparently increased infectivity. The ability of certain strains to utilize a broader range of carbon sources may confer an advantage during the commonly observed phenomenon of infection of a single host by multiple strains, or may be beneficial for their survival in nutrient-poor environments. • Only the understanding of the complex in vivo nutritional requirements of bacterial pathogens can lead to the effective design of new antimicrobials. Conclusions 30.11.2009