* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Amino Acids 14.5 * 14.8
Citric acid cycle wikipedia , lookup
Gene expression wikipedia , lookup
Signal transduction wikipedia , lookup
Interactome wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Magnesium transporter wikipedia , lookup
Ancestral sequence reconstruction wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Metalloprotein wikipedia , lookup
Western blot wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Point mutation wikipedia , lookup
Ribosomally synthesized and post-translationally modified peptides wikipedia , lookup
Peptide synthesis wikipedia , lookup
Genetic code wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Biosynthesis wikipedia , lookup
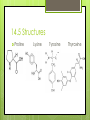
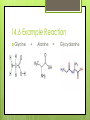
Amino Acids 14.5 – 14.8 By: Jean Turber, Kaitlin Clark & Kurstyn Pfleegor 14.5 Uncommon Amino Acids Occur in some proteins, but not all. Derived from common amino acids. Produced in a process called post-translational modification. ‾ This is the process in which protein is synthesized. Hydroxyproline & Hydroxylysine differ from their parent amino acids ‾ Their found in connective tissue proteins ‾ ‾ collagen They have hydroxyl groups on their side chains. 14.5 Uncommon Amino Acids Continued Thyroxine differs from tyrosine. ‾ Has extra iodine-containing aromatic group on the side chain. ‾ Found only in the thyroid gland. ‾ Formed by post-translational modification of tyrosine. ‾ ‾ This process produces in the protein thyroglobulin. Released as a hormone by proteolysis of thyroglobulin. 14.5 Structures Proline Lysine Tyrosine Thyroxine 14.6 How do Amino Acids Combine to Form Proteins? Amino Acid has a carboxyl group and an amino group. The –COO group of one amino acid molecule can combine with the –NH3 group of a second molecule. ─ ─ ─ ─ this reaction takes place in the cell. Produces an Amide. The two Amino Acids are joined together by an peptide bond. (the linking of two amino acids) Produces dipeptide. 14.6 Example Reaction Glycine + Alanine = Glycyalanine 14.6 Continued Dipeptide By adding more amino acids it will turn into a tripeptide, tetrapeptide ect. Chain of hundreds or thousands of amino acids make up protein that serve many functions in living organisms. The order of chain length goes by peptide (shortest), polypeptide, proteins (longest). Polypeptides contain 30-50 amino acids ─ Two amino acids combined together. the amino acids in the chain are called residues One letter or three letter abbreviations are used to represent proteins and peptides ( Ala, Gly, Lys) 14.6 Continued C-terminal amino acid is the residue with the free –COO group. N-terminal amino acid is the residue with the free NH3 group. The amino acid at the end of a peptide that has a free carboxyl group. Has a free amino group. Proteins are synthesized from N-terminal to C- terminal. 14.7 What are the Properties of Proteins? The R group are called the side chains. The 6 atoms of the peptide backbone lie on the same plane. 2 adjacent peptide bonds can rotate relative to one another. The side chains determine the physical and chemical properties of proteins. 14.7 Properties Proteins behave as zwitterions. Side chains of glutamic and aspartic acids provide acidic groups. Lysine and Arginine provide basic groups. The isoelectric point of a protein occurs at the pH equal number of positive and negative charges. Any pH above the isoelectric point the molecules have a negative charge. Any pH below the isoelectric point the molecules have a positive charge. 14.7 Properties Continued Hemoglobin has equal numbers of acidic and basic groups. has a pH of 6.8 Serum albumin has more acidic groups than basic groups. pH of 4.9 Proteins act as buffers in the blood. Water solubility depends on repulsive forces between like charges. 14.7 Properties Continued Protein molecules have a charge that causes them to repel one another. When there are no repulsive forces protein molecules clump together to form two or more molecules, reducing there solubility. Primary structure describes the linear sequence of amino acids in the polypeptide chain. Secondary structure refers to repeating patterns. Tertiary structure describes the overall conformation of the polypeptide chain. Quarternary structure applies mainly to proteins containing more than one poly peptide chain. 14.8 What is the Primary Structure of Proteins? The primary structure consists of a sequence of amino acids in a chain Decarboxylation Loss of CO2 Each protein has its own unique sequence of amino Naming them starts at the N-terminal end The primary structure determines the native secondary and tertiary structures 14.8 Protein Structures 14.8 Continued Particular sequences of amino acids allow the whole chain to fold up or curl up Different sequences may or may not affect the way it functions Cytochrome In humans, chimpanzees, sheep, and other animals Humans and chimpanzees have the same sequence 14.8 Continued People with diabetes use insulin from cows, sheep, and hogs The difference in insulin is in the 8,9, and 10 positions of the A-chain and the C-terminal position of the B-chain Not as affective as human insulin Human insulin is produced from bacteria Some people can be allergic to bovine insulin 14.8 Hormones Two peptide hormones Oxytocin Vasopressin Identical structures Disulfide bonds Difference in the amino acids in positions 2 & 7 Vasopressin increases the amount of water reabsorbed by the kidneys and raises blood pressure 14.8 Hormones Continued Oxytocin affects the contracts of the uterus at child birth In the blood protein hemoglobin a change in any one of the 146 amino acids is enough to cause sickle cell anemia The sequence of an amino acids very important Sequence of 10,000 protein and peptide molecules have been determined 14.8 Hormone Structures Oxytocin Vasopressin