* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download PDF
Epigenetics in learning and memory wikipedia , lookup
Epigenetics in stem-cell differentiation wikipedia , lookup
Epigenetics of human development wikipedia , lookup
Epigenetics of diabetes Type 2 wikipedia , lookup
Designer baby wikipedia , lookup
Microevolution wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Nutriepigenomics wikipedia , lookup
Gene expression programming wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Gene expression profiling wikipedia , lookup
Gene therapy of the human retina wikipedia , lookup
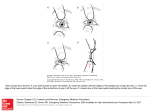
REVIEW 1455 Development 134, 1455-1463 (2007) doi:10.1242/dev.000117 Foxe view of lens development and disease Olga Medina-Martinez and Milan Jamrich* Introduction The classical studies of lens development (Lewis, 1904; Spemann, 1901) led to several important insights into the processes of induction and tissue specification. Since lens development has many features in common with the development of other placodal structures, such as the anterior pituitary, otic vesicle, olfactory epithelium, trigeminal and epibranchial placodes, studies of lens induction have implications beyond that of eye research. In this review, we focus on the early stages of lens development, during which the lens-forming gene network is established. We highlight the genes involved in this process and discuss the phenotypic consequences that mutations in the individual components of this network have on rodent and human eye development and diseases. This review is structured around the regulation and function of the transcription factor Foxe3, as mutations in this gene have been shown to cause human and rodent lens diseases. Lens induction: the tissues involved The vertebrate lens is formed from the lateral surface ectoderm of the head under the influence of the anterior neuroectoderm that is destined to become the retina (Fig. 1A). Upon contacting the evaginating optic vesicle, the surface ectoderm thickens and forms a lens placode (Fig. 1B). During subsequent stages of development, the lens placode invaginates and forms a lens vesicle (Fig. 1C,D). The cells at the posterior of the vesicle begin to differentiate and elongate to form the lens fiber cells (Fig. 1E). The lens fiber cells eventually lose their nuclei and become transcriptionally silent. By contrast, the cells at the anterior of the vesicle undergo only limited differentiation and form the anterior lens epithelium. This epithelium remains proliferatively active throughout the life of the individual, slowly adding new lens fibers to the pre-existing lens. The requirement for the presence of the optic vesicle in lens induction is not entirely without controversy. Whereas in higher vertebrates the requirement for signaling from the optic vesicle during lens induction is well demonstrated (Brownell et al., 2000; Kamachi et al., 1998; Mathers et al., 1997; Porter et al., 1997; Swindell et al., 2006), in lower vertebrates, such as frog and salmon, Department of Molecular and Cellular Biology, and Department of Molecular and Human Genetics, Baylor College of Medicine, One Baylor Plaza, Houston, TX 77030, USA. *Author for correspondence (e-mail: [email protected]) lens formation without the formation of the retina has been reported (Mencl, 1903; Spemann, 1912). How the lens develops in these species in the absence of the retina is not understood. The nature of this problem can best be demonstrated by the example of lens formation in the zebrafish retinal homeobox gene 3 (rx3; chokh) mutant, which forms a lens without ever forming a retina (Loosli et al., 2003). In zebrafish, rx3 is necessary for the morphogenesis of the optic vesicle. Although it is true that the optic vesicle does not form in rx3 mutants, a part of the neuroectoderm (brain) displays retina-specific gene expression (Loosli et al., 2003). This gene expression is likely to be responsible for the induction of lens formation in this mutant. This example demonstrates the need for molecular analyses of the processes involved in ‘retina-free’ lens induction. Although there has been great progress in the analysis of lens formation during the last decade, it is still not fully understood how developmental processes and genes function during lens development and disease. For this reason, the study of the function and regulation of genes expressed during the early stages of lens development is of great interest. As expected, many genes have already been identified that are expressed during early lens development, but most of them are also expressed in other tissues of the embryo, without lenses forming at these locations. This observation has led to the conclusion that interactions among several gene products are necessary for the initiation of lens formation. Foxe3 and lens development One of the first genes to display lens-specific expression in the mouse eye is the Fox gene Foxe3. Foxe3 encodes a DNA-binding transcription factor that has an onset of expression that is coincidental with the formation of the lens placode (Blixt et al., 2000; Brownell et al., 2000). Foxe3 was initially isolated on the basis of its similarity to the Xenopus gene Xlens1 (also called FoxE4), which is the earliest specific marker of lens development in Xenopus (Kenyon et al., 1999). In mouse, Foxe3 is initially expressed in the undifferentiated lens placode, and later its expression persists in the relatively undifferentiated anterior lens epithelium, but not in the differentiating lens fiber cells. The first clues about the crucial function of Foxe3 in lens development came from chromosomemapping experiments, which placed this gene on chromosome 4 (Blixt et al., 2000; Brownell et al., 2000), in the vicinity of the dysgenetic lens (dyl) locus (Sanyal et al., 1986). Mice carrying the dyl mutation display abnormal lens development (Fig. 2B). Sequencing of Foxe3 from the dyl strain of mice revealed the existence of two mutations in the DNA-binding domain of Foxe3, which reduce the ability of the Foxe3 protein to interact with DNA (Blixt et al., 2000; Brownell et al., 2000). In a dyl homozygous mutant, this reduced capability of Foxe3 to bind to DNA results in the formation of smaller lenses, in which the anterior lens epithelium does not separate from the cornea (keratolenticular adhesion). Later in development, some lens cells get extruded owing to intraocular pressure, and the remaining lens develops a cataract (Sanyal and Hawkins, 1979; Sanyal et al., 1986). Heterozygous dyl mice display corneal and lenticular defects, with a variable degree of penetrance (Ormestad et al., 2002). DEVELOPMENT The recent identification of a mutation in Foxe3 that causes congenital primary aphakia in humans marks an important milestone. Congenital primary aphakia is a rare developmental disease in which the lens does not form. Previously, Foxe3 had been shown to play a crucial role in vertebrate lens formation and this gene is one of the earliest integrators of several signaling pathways that cooperate to form a lens. In this review, we highlight recent advances that have led to a better understanding of the developmental processes and gene regulatory networks involved in lens development and disease. 1456 REVIEW Development 134 (8) A HSE NE B C D E LF R OV LP LV ALE In mice with a targeted deletion of both alleles of Foxe3 (MedinaMartinez et al., 2005), the cells of the anterior lens epithelium cease to proliferate prematurely and the lens is smaller, sometimes almost absent (Fig. 2D). The anterior lens epithelium fails to separate from the cornea (Fig. 2C), and the differentiating fiber cells do not lose their nuclei; they also do not elongate properly. Eventually, severe vacuolization takes place and a cataract develops. As a consequence of abnormal lens development, the retina shrinks and displays abnormal folding. Corneal dystrophy frequently accompanies this condition. Why is lens development abnormal without Foxe3 function? The Foxe3 mutant lens undergoes several abnormal morphological changes, such as a reduction in size, keratolenticular adhesion, cataract formation and altered differentiation of fiber cells. To link morphological changes to molecular events is a daunting task when the mutated gene encodes a transcription factor, as its function might affect the expression of hundreds of genes (Chauhan et al., 2002). With this in mind, we will attempt to explain the morphological changes that occur in lenses with mutated Foxe3, on the basis of molecular changes that have been detected in the lenses of Foxe3 mutant mice. One of the changes observed in mice with mutant or absent Foxe3 protein is the smaller size of the lens placode, which leads to a smaller mature lens. In Foxe3 mouse mutants, the anterior lens epithelium shows reduced proliferation, as measured by BrdU incorporation (Blixt et al., 2000; Medina-Martinez et al., 2005). This reduced proliferation might be partially due to the abnormal expression of the cyclin-dependent kinase inhibitor Cdkn1c (Blixt et al., 2000). Cdkn1c blocks cell cycle progression and is normally not expressed in the anterior lens epithelium of the wild-type mouse lens. However, in Foxe3 mutants, its expression extends into the posterior part of the lens epithelium, which is typically the most active zone of proliferation. As a result, the proliferation in this region is strongly reduced. The initial cause of this ectopic expression of Cdkn1c appears to be the deregulation of expression of Prox1, which is the mouse homolog of the Drosophila homeobox gene prospero (Oliver et al., 1993). Prox1 expression is barely detectable in the anterior lens epithelium of the wild-type lens, which transcribes high levels of Foxe3 mRNA. However, in Foxe3 mutants, high levels of Prox1 mRNA are present in the posterior part of the lens epithelium (Blixt et al., 2000). This high level of Prox1 is presumably responsible for the upregulation of Cdkn1c, as Prox1 seems to be the key regulator of Cdkn1c expression (Wigle et al., 1999). Although the most anterior epithelial cells are not affected by the premature expression of Prox1, they do stop proliferating, suggesting that another mechanism is involved in the control of proliferation of anterior epithelial cells. This regulation of proliferation might be mediated by the platelet-derived growth factor alpha (Pdgf␣), which is secreted by the ciliary body, iris epithelium and corneal endothelium. This growth factor is released into the anterior chamber and binds to the platelet-derived growth factor alpha receptor (Pdgfr␣), which is expressed in the lens epithelium. Upon binding of Pdgf␣ to its receptor, proliferation is induced (Reneker and Overbeek, 1996). In Foxe3 mutants, the expression of Pdgfr␣ is strongly reduced (Blixt et al., 2000). This reduced expression of Pdgfr␣ most likely contributes to the reduced proliferation in the anterior lens epithelium. Surprisingly, in zebrafish in which foxe3 expression has been reduced by a foxe3 morpholino, proliferation in the lens, as measured by the expression of proliferating cell nuclear antigen (pcna), is increased rather than decreased (Shi et al., 2006). However, as in mouse Foxe3 mutants, the size of the lens in zebrafish morphants is smaller than in wild-type embryos. It is not known whether the opposite effect of Foxe3 on proliferation in mouse and zebrafish is a result of an evolutionary altered function, or a discrepancy that has arisen from the use of different diagnostic methods to measure cell proliferation. Another abnormal feature of lenses in Foxe3 mouse mutants is the lack of separation of the anterior lens epithelium from the cornea, which might be due to the lack of apoptosis in the lens stalk. Cell death by apoptosis has been implicated as a mechanism in the separation of the lens from the cornea (Ozeki et al., 2001). Alternatively, the lack of separation of the anterior lens epithelium from the cornea might be due to the abnormal expression of cell adhesion molecules, such as the N- and E-cadherins, in the anterior lens epithelium. The published data on the expression of cadherins and the apoptosis of the lens stalk in dyl mutants is limited (Blixt et al., 2000) and cannot be easily used to determine if any of these two processes is responsible for the lack of separation of the lens from the cornea. DEVELOPMENT Fig. 1. Schematic of vertebrate lens development. (A) Morphogenesis of the lens begins when the evaginating optic vesicle (OV), which derives from the neuroectoderm (NE, blue), contacts the head surface ectoderm (HSE, yellow). (B) Upon this contact, the HSE thickens and forms a lens placode (LP). (C,D) The optic cup subsequently forms the retina (R) and the lens placode invaginates and forms a lens vesicle (LV). (E) Once the vesicle is formed, the lens cells in the posterior half of the vesicle elongate and form the lens fiber cells (LF). By contrast, the cells in the anterior of the lens vesicle remain as a monolayer and form the anterior lens epithelium (ALE). Modified with permission from Lovicu and McAvoy (Lovicu and McAvoy, 2005). Development 134 (8) REVIEW 1457 Fig. 2. A comparison of wild-type and Foxe3 mutant mouse eyes. (A) Hematoxylin-Eosin (HE)-stained coronal section of a P14 wild-type mouse eye showing a lens stained in pink. (B) An HE-stained coronal section of a P1 eye from a dyl mouse showing an abnormal small lens, cornea and retina (blue). (C,D) Coronal sections of eyes from P14 Foxe3–/– mice showing abnormal features of the lens, cornea and retina. Note the rudimentary lens (arrowed) in D. (E) Cerebral magnetic resonance imaging (MRI) of a 3-year-old human subject with a mutated FOXE3 gene, showing absence of the lens. (F) An HE-stained section through the eye of a 3-month-old child with a mutated FOXE3 gene, showing the absence of the lens (arrow). The asterisk indicates the empty cavity, most likely corresponding to the vitreous. c, cornea; l, lens; on, optic nerve; r, retina. A,C,D are reproduced with permission from Medina-Martinez et al. (Medina-Martinez et al., 2005); B is from I. Brownell and M.J., unpublished; E,F are reproduced with permission from Valleix et al. (Valleix et al., 2006). Somewhat later, during lens differentiation, another abnormal feature becomes apparent in Foxe3 mouse mutants. The differentiating lens fiber cells do not lose their nuclei, they do not assume a fiber-like shape and the lens develops a cataract. The loss of nuclei, which is typical of lens fiber differentiation, is absent in Foxe3 mutants and can be explained by the involvement of Foxe3 in the regulation of the DNase II-like acid DNase [Dlad; also known as deoxyribonuclease II beta (Dnase2)]. This nuclease is responsible for the degradation of the nuclear DNA during lens differentiation. In its absence, the DNA is not degraded and the nuclei are not lost (Nishimoto et al., 2003). In Foxe3-null mice, there is a significant downregulation of Dlad. It is likely that as a consequence of this Dlad downregulation, the nuclei do not get eliminated in mutant lens cells (Medina- FOXE3 and human disease After the initial observations that mutations in Foxe3 cause abnormal lens development in mice, it was quickly realized that the ocular defects in Foxe3 mutant mice resembled conditions frequently encountered in humans. The analysis of DNA from patients with Peters’ anomaly (Peters, 1906; Smith and Velzeboer, 1975) identified a frameshift mutation in FOXE3 as one of the causes of this abnormal condition (Semina et al., 2001). Peters’ anomaly is a congenital disease that frequently manifests as central corneal opacity, keratolenticular adhesion and, sometimes, anterior polar cataract. This condition can be caused by mutations in PAX6 (Hanson et al., 1994), PITX2 (Doward et al., 1999) or CYP1B1 (Vincent et al., 2006; Vincent et al., 2001), but in most cases the genetic basis is unknown. Further evidence for the role of FOXE3 in Peters’ anomaly was provided by Ormestad and colleagues (Ormestad et al., 2002), who identified a heterozygous individual in which a rare, non-conservative substitution in FOXE3 resulted in Peters’ anomaly. Finally, it was found recently that a mutation in FOXE3 in humans is one cause of congenital primary aphakia (Fig. 2E,F) (Valleix et al., 2006). Human aphakia is a rare congenital eye disorder in which the lens is missing. In primary aphakia, lens formation does not take place and the secondary ocular defects, including a complete aplasia of the anterior segment of the eye, are considered to be the result of the absence of the lens. In secondary aphakia, lens formation does take place, but the lens degenerates and is resorbed perinatally. For this reason, the ocular defects in secondary aphakia tend to be less severe than in primary aphakia. The features of congenital primary aphakia in humans, described by Valleix and co-workers (Valleix et al., 2006), resemble the extreme phenotype of Foxe3-null mice, in which only very small, undifferentiated lenses develop (Fig. 2D; Medina-Martinez et al., 2005). The phenotype in humans appears to be somewhat more pronounced, as lenses are totally absent and other eye structures, including the iris, ciliary body and trabecular meshwork, are missing. However, it is difficult to make a generalization, as the data on congenital primary aphakia are based on only one family. In addition, the direct comparison of human and DEVELOPMENT Martinez et al., 2005). Another contributing factor to the cataract formation in Foxe3 mutants might be the altered expression of ␣A-crystallin. In the wild-type lens, lens fiber cells express high levels of crystallins, which can represent more than 90% of the total protein in the cell. ␣A-crystallin constitutes about 17% of the total protein in the cell. Studies into crystallin structure and regulation have provided some important insights into their evolution, as well as into gene sharing in general. It was found that crystallins are not lens-specific proteins, but rather are proteins that are also utilized by other cells of the body, but for different functions (Piatigorsky, 1998; Wistow and Piatigorsky, 1987). For example, it was found that ␣A-crystallin is a small heat-shock protein (Ingolia and Craig, 1982) that can act as a molecular chaperone (Horwitz, 1992; Jakob et al., 1993). More recently, analysis of the zebrafish mutant cloche, which develops a cataract, revealed that a downregulation of ␣A-crystallin expression leads to the insolubility of ␥-crystallin and to an opaque lens (Goishi et al., 2006). In Foxe3 mutants, the transcription of ␣A-crystallin is altered (Blixt et al., 2000; Brownell et al., 2000; Medina-Martinez et al., 2005), raising the possibility that this misregulation of ␣Acrystallin expression leads to a reduced solubility of ␥-crystallin, which might contribute to cataract formation. The inability of lens fiber cells in Foxe3 mutants to assume the fiber-like cell shape is unexplained at present. mouse diseases is not a trivial task, as the phenotype of a disease in mice sometimes varies according to the genetic background of the strain (Blixt et al., 2006). Upstream of Foxe3 Whereas mutations in Foxe3 cause severe abnormalities in lens development, there are several genes that are expressed prior to Foxe3 that are necessary for lens formation. Some of these genes are expressed in head surface ectoderm, but also in other tissues. For example, the homeobox-containing gene Pax6 is expressed during early lens development, and mutations in this gene lead to eye disorders, known as small eye (sey) syndrome in mice and rats (Fujiwara et al., 1994; Hill et al., 1991), and aniridia and Peters’ anomaly in humans (Glaser et al., 1994; Hanson et al., 1994; Jordan et al., 1992; Ton et al., 1991). However, Pax6 is not only expressed in the superficial head ectoderm, from which the lens is derived, but also in the neuroectoderm, from which the retina is derived. The expression in the superficial head ectoderm seems to be crucial for lens formation, as Pax6-deficient superficial head ectoderm does not form a lens when transplanted onto a wild-type optic vesicle (Fujiwara et al., 1994). Furthermore, the lens-specific ablation of Pax6 expression in mice using a floxed allele of Pax6 crossed to a lens-specific Le-Cre, results in a lack of lens formation (AsheryPadan et al., 2000). Whether Pax6-deficient neuroectoderm is able to induce lens formation is not entirely clear. Although Fujiwara and colleagues (Fujiwara et al., 1994) showed that the wild-type ectoderm can induce lens formation when transplanted on the Pax6deficient neuroectoderm, they also observed the first morphological signs of lens induction in the wild-type head ectoderm before the transplantation was performed. Therefore, it is not certain whether lens induction takes place in the absence of Pax6 in the neuroectoderm. A genetic ablation of Pax6 using Pax6flox and a retinal neuroectoderm-specific Cre, such as Rx-Cre (Swindell et al., 2006), should provide a definitive answer to this question. Furthermore, it is also unclear to what degree the Pax6 function in the neuroectoderm is required for the normal differentiation of the lens. The rat recombination experiments of Fujiwara and colleagues (Fujiwara et al., 1994) showed that a significant degree of lens differentiation takes place in the absence of Pax6 expression in the neuroectoderm. However, in chick in which Pax6 function in the neuroectoderm was eliminated by the injection of either a Pax6specific morpholino or Pax6 dominant-negative construct, the differentiation of the lens did not proceed normally (Canto-Soler and Adler, 2006; Reza and Yasuda, 2004). Interestingly, mutations in PAX6, as in FOXE3, cause Peters’ anomaly (Hanson et al., 1994). The explanation for this phenomenon is that Foxe3 appears to be regulated by Pax6 (Blixt et al., 2006; Brownell et al., 2000; Dimanlig et al., 2001), and in humans, as far as the lens is concerned, mutations in both genes seem to have a very similar clinical manifestation. Pax6 is involved in a complex regulatory network. Pax6 is autoregulated by itself (Aota et al., 2003), as well as being regulated by the Six3 and Meis homeoproteins, which are required for lens formation (Liu et al., 2006; Zhang et al., 2002). Important regulatory partners of Pax6 are the Sox proteins, which have been implicated in lens induction (Donner et al., 2006a; Kamachi et al., 1998; Kamachi et al., 2001; Kondoh et al., 2004; Koster et al., 2000). Sox2 in combination with Oct-1 (Pou2f1 – Mouse Genome Informatics) protein control the maintenance of Pax6 expression, and through this activity they control lens formation (Donner et al., 2006a). Several other genes have been suggested to be upstream regulators of Foxe3, including Mab21/1, a member of the Mab gene family, which is also essential Development 134 (8) for the development of the lens placode in mouse (Yamada et al., 2003). Mab21/1 function is necessary for Foxe3 expression (Yamada et al., 2003). Furthermore, the Smad-binding zinc-finger homeodomain transcription factor Sip1 is also essential for the activation of Foxe3 expression (Yoshimoto et al., 2005). During the activation of Foxe3, Sip1 interacts with Smad8 (also known as Smad9 in mouse), which is one of the mediators of Bmp4 signaling in vertebrates. The Bmp4 pathway has previously been demonstrated to be crucial for lens formation (Furuta and Hogan, 1998). Although a complete picture of the interactions of the different transcription factors cannot be drawn at this point, some regulatory interactions have been depicted in Fig. 3. In addition to transcription factors, several signaling pathways have been implicated in lens formation, including the Wnt signaling pathway. This signaling pathway needs to be integrated into any model of lens development owing to the observation that when catenin function is eliminated in the Pax6-expressing area in the presumptive lens ectoderm, ectopic lens-like structures develop in this location (Smith et al., 2005). This suggests that the elimination of -catenin signaling is crucial for lens formation. Consistent with this observation, -catenin loss-of-function in the lens placode does not alter lens fate (Smith et al., 2005), presumably because the catenin signaling was already eliminated in this tissue. All of these observations indicate that several independent pathways are integrated into a network responsible for the formation of a lens. How this integration of several signaling pathways works at the molecular level is not yet fully understood. A possible mechanism for the establishment of lens-specific expression was recently demonstrated by Yang and co-workers, through their studies of the ␣A-crystallin gene (Cryaa) (Yang et al., 2006). Pax6 initially binds the cis-regulatory elements of Cryaa. This binding attracts Brg1 (Smarca4 – Mouse Genome Informatics), a murine homolog of the Drosophila brahma gene (Khavari et al., 1993; Randazzo et al., 1994). Brg1 is a catalytic subunit of SWI/SNF, an evolutionary conserved class of chromatin remodeling factors (Mohrmann and Verrijzer, 2005). The partially remodeled chromatin becomes accessible to additional transcription factors, such as c-Maf (Maf – Mouse Genome Informatics) (Ishibashi and Yasuda, 2001; Yoshida et al., 1997), which facilitate the activity of further chromatin remodeling enzymes to fully open the Cryaa promoter to additional transcriptional regulators. Pre-placodal gene expression Several transcriptional regulators that are essential for lens formation, such as Pax6, Six3 and Sox2, are not only expressed in the lens placode, but also in other placodal structures. Because of their expression in multiple placodal structures, several authors have suggested that during early development a uniform, U-shaped, preplacodal field is induced that surrounds the anterior neuroectoderm (Bailey et al., 2006; Baker and Bronner-Fraser, 2001; Donner et al., 2006b; Jacobson, 1966; Kenyon et al., 1999; Schlosser and Ahrens, 2004; Torres and Giraldez, 1998). Only later is this pre-placodal field divided into individual placodes. Gene expression in the Ushaped field in the anterior non-neural ectoderm can be demonstrated by the example of Xlens1, the Xenopus functional homolog of Foxe3. During neurulation, Xlens1 has a U-shaped expression domain, with the strongest expression in the middle of the field (Fig. 4A; our unpublished observations). Later in development, its expression diminishes in the middle and becomes more prominent in the presumptive lens placodes (Fig. 4B) (Kenyon et al., 1999). Several genes display a similar U-shaped expression pattern, with the field varying slightly in size and location, indicating DEVELOPMENT 1458 REVIEW Fig. 3. Regulatory interactions during lens formation in the head surface ectoderm and lens. (A) Schematic depicting selected transcription factors important for lens formation that are expressed in the mouse head surface ectoderm. These interactions help specify the activation of Foxe3 in the lens placode. (B) Roles of Foxe3 in the development and differentiation of the lens (orange). Feedback loops are not depicted here and arrows between genes do not necessarily imply direct regulatory interactions. that some regionalization of this domain already takes place during gastrulation (for a review, see Schlosser, 2006). This pre-placodal field initially encompasses a large part of the anterior, lateral and ventral head surface ectoderm and gives rise to several placodal and non-placodal structures. The establishment of this field was suggested to be the first step towards the establishment of lens competence in Xenopus (Servetnick and Grainger, 1991). A part of this field is called the pre-lens ectoderm (Ashery-Padan et al., 2000; Schlosser, 2006; Schlosser and Ahrens, 2004; Williams et al., 1998). The central part of the pre-lens ectoderm forms the lens placode, whereas the lateral parts of this field form non-placodal structures. It was recently proposed, based on experiments in chick, that the entire pre-placodal region is initially specified to form a lens (Bailey et al., 2006), and therefore it is not clear how the pre-placodal field differs from the pre-lens ectoderm in terms of lens competence. Whether the model of lens induction proposed by Bailey and colleagues applies to mammals is uncertain, as it contradicts data from mouse experiments. There is substantial evidence in mice that the optic vesicle is necessary for lens induction and the initiation of lens-specific gene expression (Faber et al., 2001; Furuta and Hogan, 1998; Wawersik et al., 1999). Furthermore, mice deficient in Rx (Rax – Mouse Genome Informatics) function, which have no retinal REVIEW 1459 Fig. 4. Expression of Xlens1 and Foxe3 during lens formation in Xenopus and mouse. (A) Anterior view of Xlens1 expression in the U-shaped region of Xenopus neurula (our unpublished observation). (B) Expression of Xlens1 in the lens placodes of a Xenopus tadpole (Kenyon et al., 1999). (C) The earliest expression of Foxe3 in the lens placode of an E9.0 mouse embryos (arrow). Reproduced with permission from Blixt et al. (Blixt et al., 2000). (D) Expression of Foxe3 in an E12 mouse embryo. Reproduced with permission from Brownell et al. (Brownell et al., 2000). neuroectoderm, display no lens-specific gene expression and form no lenses (Bailey et al., 2004; Brownell et al., 2000; Mathers et al., 1997; Zhang et al., 2000; Zilinski et al., 2004). In these mice that lack retina and lens, other placodal structures form normally and the non-placodal structures arising from the ‘pre-lens ectoderm’ also form normally (E. Swindell and M.J., unpublished). These experiments clearly show that gene expression in the head surface ectoderm without the input of Rx-expressing retinal cells is not sufficient to induce the lens. Therefore, changes in gene expression orchestrated by the optic vesicle appear to be essential for the establishment of lens fate in mice. For this reason, an important task for the future will be to establish the gene expression pattern in the head surface ectoderm in the absence of the retina, and compare it with that in the presence of the retina. Only then will we be able to distinguish changes in gene expression that are due to general cranio-facial patterning from those specifically needed to make a lens. There are several possibilities to explain these discrepancies and one of them is to postulate that evolutionary changes have occurred in the specification of placodal structures. One piece of evidence supporting this hypothesis is the expression pattern of Foxe3. Whereas Xlens1 is initially expressed in a U-shaped field that surrounds the anterior neural ectoderm (Fig. 4A) (Kenyon et al., 1999; Zilinski et al., 2004), Foxe3 in mice is not expressed in a Ushaped domain. From the earliest onset, Foxe3 is expressed in two distinctly separate fields (Fig. 4C) (Blixt et al., 2000), indicating that different mechanisms for lens specification exist in Xenopus and mouse. Although the differences in the expression patterns of these DEVELOPMENT Development 134 (8) two Fox genes are striking, the significance of the Xlens1 U-shaped expression with respect to lens formation is not understood. Since cell fate experiments were not performed on the cells in the Ushaped Xlens1 expression area, it is not clear whether these cells generate all or most of the cells of the lens placode. It is possible that the expression of Xlens1 in the U-shaped region and in the lens placodes reflect two independent processes. It is also possible that the early U-shaped expression of certain genes is not necessary for lens formation. It is important to remember that even though the expression of Xlens1 or Foxe3 in the eye is lens-specific, these genes are expressed in other tissues in the embryo that do not form a lens (Blixt et al., 2000; Brownell et al., 2000; Yu et al., 2002). For this reason, it is not necessarily valid to use the expression of a certain gene as an indicator of the formation of a specific structure. Although additional experiments will be needed to determine the role of this early U-shaped expression in lens formation, it is nonetheless certain that a lens placode eventually develops that has a different appearance and a different gene expression profile than the surrounding surface ectoderm or other placodal structures. The question of which developmental steps make the lens cells become different from their neighbors is central to lens research, but the answer to this question remains unknown. As mentioned previously, it appears that the mechanism of lens induction is not wholly conserved in all vertebrate species. To find evolutionary changes in lens induction might not be entirely surprising, as lens differentiation in various species displays significant variations. For example, whereas in most species the lens undergoes invagination, in zebrafish the lens is generated through delamination (Soules and Link, 2005). Furthermore, different mechanisms of lens suture formation in different species indicate that the lens-forming network has an inherent flexibility to produce correct lenses in each species (Kuszak et al., 2004). Therefore, although most of the components in lens formation are conserved (Donner et al., 2006b), their interactions might be modified to varying degrees in different species. As such, the use of a single model to explain lens formation in all species might be counterproductive for the better understanding of lens formation in each separate species. Ectopic lens formation Although most of the evidence suggests that a specific gene network is required to initiate lens development, gene networks present in other tissues can be manipulated to form a lens. For example, overexpression of Pax6 can lead to ectopic lens formation in Xenopus (Chow et al., 1999). However, Pax6 can induce lens formation more easily in the head ectoderm than in the trunk ectoderm of Xenopus (Chow et al., 1999), demonstrating that some gene networks can be more easily modified to form a lens than others. The ectopic expression of Sox3 can induce ectopic lens formation in the head surface ectoderm of Medaka (Koster et al., 2000), and ectopic expression of six3, which is normally expressed in the lens placode, leads to the formation of a lens in the ear placode of zebrafish (Oliver et al., 1996). The molecular process of changing one placodal structure into another can be demonstrated by the formation of the zebrafish anterior pituitary gland. The anterior pituitary gland is a placode-derived structure that, in its early stages, seems to have the same gene expression pattern as the lens placode. This common placodal area expresses the transcription factor pitx3 and can form either pituitary or lens (Dutta et al., 2005; Shi et al., 2005; Zilinski et al., 2005). To form the anterior pituitary gland, hedgehog signaling is required in the placodal region. In the zebrafish mutant smoothened, hedgehog signals cannot be Development 134 (8) transduced, and the pituitary precursors form an ectopic lens instead of a pituitary (Dutta et al., 2005). If sonic hedgehog (shh) is overexpressed in the lens precursors, they begin to express genes characteristic of pituitary cells (Dutta et al., 2005). Although the ability to induce ectopic lens formation is intriguing, the ectopic induction of lens-specific gene expression might not follow the normal sequence of events involved in lens formation. For example, the Maf gene family plays an important role in lens formation in several vertebrate species (Ishibashi and Yasuda, 2001; Reza et al., 2002). In Xenopus, overexpression of XmafB, a member of the Maf gene family, in animal caps leads to the activation of Xlens1 (Ishibashi and Yasuda, 2001). However, the analysis of gene expression in intact embryos shows that XmafB expression in the head ectoderm normally starts several hours after Xlens1 is activated (Ishibashi and Yasuda, 2001; Kenyon et al., 1999). Therefore, the activation of Xlens1 probably does not require XmafB during normal development. This observation suggests that the process of ectopic tissue formation can utilize an alternative mechanism for tissue specification. For example, feedback loops, which might play a minor role during normal development, could be used to activate genes that are normally upstream in the regulatory cascade. An interesting example of ectopic lens formation was recently described in chick embryos, in which the neural crest cells were surgically removed from the embryo (Bailey et al., 2006). In these embryos, ectopic lenses develop posterior to the endogenous lenses. This is presumably because the neural crest cells play an inhibitory role in lens formation and, during their absence, regions of head surface ectoderm form lenses that would normally have been prevented from doing so (Bailey et al., 2006). A prime example of the differential pliability of gene networks is the different ability of species to regenerate a lens. Whereas the lens will readily regenerate in some amphibians (Eguchi, 1967; Eguchi, 1988; Stone, 1953; Stone, 1967; Wolf, 1895), this ability is much more limited in mammals. In newts, the dorsal iris pigment epithelium can be induced to regenerate a lens by lentectomy (Del Rio-Tsonis et al., 1998; Madhavan et al., 2006; Tsonis et al., 2004). This process of lens regeneration seems to be true transdifferentiation, as fully differentiated iris cells dedifferentiate and then redifferentiate into lens cells (Del Rio-Tsonis and Tsonis, 2003). To what degree the process of lens regeneration is similar to lens induction during early development is not entirely clear at present. Since a lens forms during lens regeneration, many of the differentiation products are shared in these two processes. However, there must certainly also be differences, as the iris cells must dedifferentiate before they start the lens differentiation program. One unanswered question is to what degree must the iris cells dedifferentiate before they can start the lens-forming process? Must they acquire the same state as the head ectoderm prior to lens induction, or is there a shortcut that the dedifferentiating iris cells use to make a lens? The published evidence suggests that although there are many similarities between the temporo-spatial expression of genes during lens induction and regeneration, there are also differences, indicating that the two processes are not identical (Mizuno et al., 2002; Mizuno et al., 1999). One intriguing possibility is that during transdifferentiation the cells assume a state that is identical, or very similar, to the state of embryonic stem (ES) cells. While this possibility is under investigation, it has already been demonstrated that primate ES cells can be directed to form Pax6and ␣A-crystallin-expressing lentoid bodies after treatment with FGF2 (Ooto et al., 2003). FGF2 triggers lens regeneration in newt (Hayashi et al., 2004), and FGF molecules and their receptors are known to play a role in different aspects of lens formation (for DEVELOPMENT 1460 REVIEW reviews, see Lovicu and McAvoy, 2005; Robinson, 2006). The ability to use ES cells to generate lentoid bodies can be exploited in experiments designed to better understand lens development. Perspectives Recent research has led to the identification of developmental processes and genes that have a role in lens formation. This research has helped to identify causes of human lens diseases and has opened new avenues for possible therapeutic approaches. Foxe3 has been identified as one of the key players in lens development. How this early transcription factor modulates aspects of lens development and differentiation is not fully understood. For example, there are only a few known targets of Foxe3, and it is not known whether Foxe3 directly regulates these targets. A search for additional targets of Foxe3 is warranted. The identification of a full complement of genes that are directly regulated by Foxe3 would provide a good starting point to assemble the gene network in which Foxe3 plays a central role. The developmental processes and gene interactions upstream of Foxe3 are also poorly understood. Disparities have been reported in lens induction between different species and one of the challenges for the future is to determine which differences are due to evolutionary changes and which are due to the methodologies used to study lens induction. One of the problems associated with comparing lens induction is that the so-called pre-placodal region is not well defined in most species. Whereas in Xenopus there is a fairly detailed map of gene expression in this region (Schlosser, 2006), in mice, the expression data for this area are rudimentary at best. In addition, the function and significance of gene expression in the pre-placodal region are not clear. For example, the expression of Pax6 in the head surface ectoderm varies at different stages of development. In some stages, it encompasses practically the entire head. Several placodal and non-placodal structures develop from the Pax6 expression area. Analysis of lens formation does demonstrate the need for Pax6 expression in the head surface ectoderm, but it remains to be demonstrated that this expression is a step specifically directed towards lens formation. In many recent developmental lens studies, changes in gene expression in the head ectoderm have been monitored as a measure of the competence of this ectoderm to form a lens. Although this lens-centric view helps to identify gene expression in the head ectoderm that is necessary for lens formation, it does not distinguish between changes in gene expression that are due to general cranio-facial patterning from those changes that are specifically made to generate a lens. If we designate all developmental processes necessary for lens formation as part of the lens-forming cascade, will we not have to declare that fertilization itself is a part of this cascade? In our opinion, the important task for the future will be to establish the gene expression pattern in the head surface ectoderm in the absence of the retina, and compare it with that in the presence of the retina. Only then we will be able to distinguish changes in gene expression that are due to general cranio-facial patterning from those specifically made to induce lens formation. Several other related questions remain unanswered. For example, is the early pre-placodal region simply the anterior and lateral head surface ectoderm from which some placodal and some non-placodal structures of the head are derived? Is the complex and overlapping gene expression in the early pre-placodal region simply reflecting the fact that different signaling sources in the head neuroectoderm are very close to each other during gastrulation? Is the progressive regionalization of gene expression in the head surface ectoderm simply reflecting the progressive differentiation and morphogenesis REVIEW 1461 of the neural tube? Is the early pre-placodal head ectoderm in other species specified to form a lens, as suggested by in vitro experiments in chick? What is the difference between the pre-placodal ectoderm and the pre-lens ectoderm? In summary, although many of the important questions in lens development and regeneration remain to be answered, we have clearly reached a stage at which developmental lens research is not only improving our molecular perspective on the initial stages of lens induction, but also contributing to a better understanding of lens diseases. We thank several esteemed colleagues for their suggestions, Drs Paul Overbeek and Eric Swindell for critical reading of the manuscript, and the anonymous reviewers for their helpful comments. References Aota, S., Nakajima, N., Sakamoto, R., Watanabe, S., Ibaraki, N. and Okazaki, K. (2003). Pax6 autoregulation mediated by direct interaction of Pax6 protein with the head surface ectoderm-specific enhancer of the mouse Pax6 gene. Dev. Biol. 257, 1-13. Ashery-Padan, R., Marquardt, T., Zhou, X. and Gruss, P. (2000). Pax6 activity in the lens primordium is required for lens formation and for correct placement of a single retina in the eye. Genes Dev. 14, 2701-2711. Bailey, A. P., Bhattacharyya, S., Bronner-Fraser, M. and Streit, A. (2006). Lens specification is the ground state of all sensory placodes, from which FGF promotes olfactory identity. Dev. Cell 11, 505-517. Bailey, T. J., El-Hodiri, H., Zhang, L., Shah, R., Mathers, E. H. and Jamrich, M. (2004). Regulation of vertebrate eye development by Rx genes. Int. J. Dev. Biol. 48, 761-770. Baker, C. V. and Bronner-Fraser, M. (2001). Vertebrate cranial placodes I. Embryonic induction. Dev. Biol. 232, 1-61. Blixt, A., Mahlapuu, M., Aitola, M., Pelto-Huikko, M., Enerback, S. and Carlsson, P. (2000). A forkhead gene, FoxE3, is essential for lens epithelial proliferation and closure of the lens vesicle. Genes Dev. 14, 245-254. Blixt, A., Landgren, H., Johansson, B. R. and Carlsson, P. (2006). Foxe3 is required for morphogenesis and differentiation of the anterior segment of the eye and is sensitive to Pax6 gene dosage. Dev. Biol. 302, 218-229. Brownell, I., Dirksen, M. and Jamrich, M. (2000). Forkhead Foxe3 maps to the dysgenetic lens locus and is critical in lens development and differentiation. Genesis 27, 81-93. Canto-Soler, M. V. and Adler, R. (2006). Optic cup and lens development requires Pax6 expression in the early optic vesicle during a narrow time window. Dev. Biol. 294, 119-132. Chauhan, B. K., Reed, N. A., Yang, Y., Cermak, L., Reneker, L., Duncan, M. K. and Cvekl, A. (2002). A comparative cDNA microarray analysis reveals a spectrum of genes regulated by Pax6 in mouse lens. Genes Cells 7, 12671283. Chow, R. L., Altmann, C. R., Lang, R. A. and Hemmati-Brivanlou, A. (1999). Pax6 induces ectopic eyes in a vertebrate. Development 126, 4213-4222. Del Rio-Tsonis, K. and Tsonis, P. A. (2003). Eye regeneration at the molecular age. Dev. Dyn. 226, 211-224. Del Rio-Tsonis, K., Trombley, M. T., McMahon, G. and Tsonis, P. A. (1998). Regulation of lens regeneration by fibroblast growth factor receptor 1. Dev. Dyn. 213, 140-146. Dimanlig, P. V., Faber, S. C., Auerbach, W., Makarenkova, H. P. and Lang, R. A. (2001). The upstream ectoderm enhancer in Pax6 has an important role in lens induction. Development 128, 4415-4424. Donner, A. L., Episkopou, V. and Maas, R. L. (2006a). Sox2 and Pou2f1 interact to control lens and olfactory placode development. Dev. Biol. doi:10.1016/j.ydbio.2006.10.047. Donner, A. L., Lachke, S. A. and Maas, R. L. (2006b). Lens induction in vertebrates: variations on a conserved theme of signaling events. Semin. Cell Dev. Biol. 17, 676-685. Doward, W., Perveen, R., Lloyd, I. C., Ridgway, A. E., Wilson, L. and Black, G. C. (1999). A mutation in the RIEG1 gene associated with Peters’ anomaly. J. Med. Genet. 36, 152-155. Dutta, S., Dietrich, J. E., Aspock, G., Burdine, R. D., Schier, A., Westerfield, M. and Varga, Z. M. (2005). pitx3 defines an equivalence domain for lens and anterior pituitary placode. Development 132, 1579-1590. Eguchi, G. (1967). In vitro analyses of Wolffian lens regeneration: differentiation of the regenerating lens rudiment of the newt, Triturus pyrrhogaster. Embryologia Nagoya 9, 246-266. Eguchi, G. (1988). Cellular and molecular background of wolffian lens regeneration. Cell Differ. Dev. 25, S147-S158. Faber, S. C., Dimanlig, P., Makarenkova, H. P., Shirke, S., Ko, K. and Lang, R. A. (2001). Fgf receptor signaling plays a role in lens induction. Development 128, 4425-4438. DEVELOPMENT Development 134 (8) Fujiwara, M., Uchida, T., Osumi-Yamashita, N. and Eto, K. (1994). Uchida rat (rSey): a new mutant rat with craniofacial abnormalities resembling those of the mouse Sey mutant. Differentiation 57, 31-38. Furuta, Y. and Hogan, B. L. (1998). BMP4 is essential for lens induction in the mouse embryo. Genes Dev. 12, 3764-3775. Glaser, T., Jepeal, L., Edwards, J. G., Young, S. R., Favor, J. and Maas, R. L. (1994). PAX6 gene dosage effect in a family with congenital cataracts, aniridia, anophthalmia and central nervous system defects. Nat. Genet. 7, 463-471. Goishi, K., Shimizu, A., Najarro, G., Watanabe, S., Rogers, R., Zon, L. I. and Klagsbrun, M. (2006). {alpha}A-crystallin expression prevents {gamma}-crystallin insolubility and cataract formation in the zebrafish cloche mutant lens. Development 133, 2585-2593. Hanson, I. M., Fletcher, J. M., Jordan, T., Brown, A., Taylor, D., Adams, R. J., Punnett, H. H. and van Heyningen, V. (1994). Mutations at the PAX6 locus are found in heterogeneous anterior segment malformations including Peters’ anomaly. Nat. Genet. 6, 168-173. Hayashi, T., Mizuno, N., Ueda, Y., Okamoto, M. and Kondoh, H. (2004). FGF2 triggers iris-derived lens regeneration in newt eye. Mech. Dev. 121, 519526. Hill, R. E., Favor, J., Hogan, B. L., Ton, C. C., Saunders, G. F., Hanson, I. M., Prosser, J., Jordan, T., Hastie, N. D. and van Heyningen, V. (1991). Mouse small eye results from mutations in a paired-like homeobox- containing gene. Nature 354, 522-525. Horwitz, J. (1992). Alpha-crystallin can function as a molecular chaperone. Proc. Natl. Acad. Sci. USA 89, 10449-10453. Ingolia, T. D. and Craig, E. A. (1982). Four small Drosophila heat shock proteins are related to each other and to mammalian alpha-crystallin. Proc. Natl. Acad. Sci. USA 79, 2360-2364. Ishibashi, S. and Yasuda, K. (2001). Distinct roles of maf genes during Xenopus lens development. Mech. Dev. 101, 155-166. Jacobson, A. G. (1966). Inductive processes in embryonic development. Science 152, 25-34. Jakob, U., Gaestel, M., Engel, K. and Buchner, J. (1993). Small heat shock proteins are molecular chaperones. J. Biol. Chem. 268, 1517-1520. Jordan, T., Hanson, I., Zaletayev, D., Hodgson, S., Prosser, J., Seawright, A., Hastie, N. and van Heyningen, V. (1992). The human PAX6 gene is mutated in two patients with aniridia. Nat. Genet. 1, 328-332. Kamachi, Y., Uchikawa, M., Collignon, J., Lovell-Badge, R. and Kondoh, H. (1998). Involvement of Sox1, 2 and 3 in the early and subsequent molecular events of lens induction. Development 125, 2521-2532. Kamachi, Y., Uchikawa, M., Tanouchi, A., Sekido, R. and Kondoh, H. (2001). Pax6 and SOX2 form a co-DNA-binding partner complex that regulates initiation of lens development. Genes Dev. 15, 1272-1286. Kenyon, K. L., Moody, S. A. and Jamrich, M. (1999). A novel fork head gene mediates early steps during Xenopus lens formation. Development 126, 51075116. Khavari, P. A., Peterson, C. L., Tamkun, J. W., Mendel, D. B. and Crabtree, G. R. (1993). BRG1 contains a conserved domain of the SWI2/SNF2 family necessary for normal mitotic growth and transcription. Nature 366, 170-174. Kondoh, H., Uchikawa, M. and Kamachi, Y. (2004). Interplay of Pax6 and SOX2 in lens development as a paradigm of genetic switch mechanisms for cell differentiation. Int. J. Dev. Biol. 48, 819-827. Koster, R. W., Kuhnlein, R. P. and Wittbrodt, J. (2000). Ectopic Sox3 activity elicits sensory placode formation. Mech. Dev. 95, 175-187. Kuszak, J. R., Zoltoski, R. K. and Tiedemann, C. E. (2004). Development of lens sutures. Int. J. Dev. Biol. 48, 889-902. Lewis, W. H. (1904). Experimental studies on the development of the eye in amphibia. III. On the origin and the differentiation of the lens. Am. J. Anat. 6, 473-509. Liu, W., Lagutin, O. V., Mende, M., Streit, A. and Oliver, G. (2006). Six3 activation of Pax6 expression is essential for mammalian lens induction and specification. EMBO J. 25, 5383-5395. Loosli, F., Staub, W., Finger-Baier, K. C., Ober, E. A., Verkade, H., Wittbrodt, J. and Baier, H. (2003). Loss of eyes in zebrafish caused by mutation of chokh/rx3. EMBO Rep. 4, 894-899. Lovicu, F. J. and McAvoy, J. W. (2005). Growth factor regulation of lens development. Dev. Biol. 280, 1-14. Madhavan, M., Haynes, T. L., Frisch, N. C., Call, M. K., Minich, C. M., Tsonis, P. A. and Del Rio-Tsonis, K. (2006). The role of Pax-6 in lens regeneration. Proc. Natl. Acad. Sci. USA 103, 14848-14853. Mathers, P. H., Grinberg, A., Mahon, K. A. and Jamrich, M. (1997). The Rx homeobox gene is essential for vertebrate eye development. Nature 387, 603607. Medina-Martinez, O., Brownell, I., Amaya-Manzanares, F., Hu, Q., Behringer, R. R. and Jamrich, M. (2005). Severe defects in proliferation and differentiation of lens cells in Foxe3 null mice. Mol. Cell. Biol. 25, 8854-8863. Mencl, E. (1903). Ein Fall von beiderseitiger Augenlinsenausbildung während der Abwesenheit von Augenblasen. Dev. Genes Evol. 16, 328-339. Mizuno, N., Mochii, M., Takahashi, T. C., Eguchi, G. and Okada, T. S. (1999). Development 134 (8) Lens regeneration in Xenopus is not a mere repeat of lens development, with respect to crystallin gene expression. Differentiation 64, 143-149. Mizuno, N., Agata, K., Sawada, K., Mochii, M. and Eguchi, G. (2002). Expression of crystallin genes in embryonic and regenerating newt lenses. Dev. Growth Differ. 44, 251-256. Mohrmann, L. and Verrijzer, C. P. (2005). Composition and functional specificity of SWI2/SNF2 class chromatin remodeling complexes. Biochim. Biophys. Acta 1681, 59-73. Nishimoto, S., Kawane, K., Watanabe-Fukunaga, R., Fukuyama, H., Ohsawa, Y., Uchiyama, Y., Hashida, N., Ohguro, N., Tano, Y., Morimoto, T. et al. (2003). Nuclear cataract caused by a lack of DNA degradation in the mouse eye lens. Nature 424, 1071-1074. Oliver, G., Sosa-Pineda, B., Geisendorf, S., Spana, E. P., Doe, C. Q. and Gruss, P. (1993). Prox 1, a prospero-related homeobox gene expressed during mouse development. Mech. Dev. 44, 3-16. Oliver, G., Loosli, F., Koster, R., Wittbrodt, J. and Gruss, P. (1996). Ectopic lens induction in fish in response to the murine homeobox gene Six3. Mech. Dev. 60, 233-239. Ooto, S., Haruta, M., Honda, Y., Kawasaki, H., Sasai, Y. and Takahashi, M. (2003). Induction of the differentiation of lentoids from primate embryonic stem cells. Invest. Ophthalmol. Vis. Sci. 44, 2689-2693. Ormestad, M., Blixt, A., Churchill, A., Martinsson, T., Enerback, S. and Carlsson, P. (2002). Foxe3 haploinsufficiency in mice: a model for Peters’ anomaly. Invest. Ophthalmol. Vis. Sci. 43, 1350-1357. Ozeki, H., Ogura, Y., Hirabayashi, Y. and Shimada, S. (2001). Suppression of lens stalk cell apoptosis by hyaluronic acid leads to faulty separation of the lens vesicle. Exp. Eye Res. 72, 63-70. Peters, A. (1906). Uber angeboren Defektbildung der Descementschen Membran. Klin. Monatsbl. Augenheilkd. 44, 27-40. Piatigorsky, J. (1998). Gene sharing in lens and cornea: facts and implications. Prog. Retin. Eye Res. 17, 145-174. Porter, F. D., Drago, J., Xu, Y., Cheema, S. S., Wassif, C., Huang, S. P., Lee, E., Grinberg, A., Massalas, J. S., Bodine, D. et al. (1997). Lhx2, a LIM homeobox gene, is required for eye, forebrain, and definitive erythrocyte development. Development 124, 2935-2944. Randazzo, F. M., Khavari, P., Crabtree, G., Tamkun, J. and Rossant, J. (1994). brg1: a putative murine homologue of the Drosophila brahma gene, a homeotic gene regulator. Dev. Biol. 161, 229-242. Reneker, L. W. and Overbeek, P. A. (1996). Lens-specific expression of PDGF-A alters lens growth and development. Dev. Biol. 180, 554-565. Reza, H. M. and Yasuda, K. (2004). The involvement of neural retina pax6 in lens fiber differentiation. Dev. Neurosci. 26, 318-327. Reza, H. M., Ogino, H. and Yasuda, K. (2002). L-Maf, a downstream target of Pax6, is essential for chick lens development. Mech. Dev. 116, 61-73. Robinson, M. L. (2006). An essential role for FGF receptor signaling in lens development. Semin. Cell Dev. Biol. 17, 726-740. Sanyal, S. and Hawkins, R. K. (1979). Dysgenetic lens (dyl) – a new gene in the mouse. Invest. Ophthalmol. Vis. Sci. 18, 642-645. Sanyal, S., Van Nie, R., De Moes, J. and Hawkins, R. K. (1986). Map position of dysgenetic lens (dyl) locus on chromosome 4 in the mouse. Genet. Res. 48, 199200. Schlosser, G. (2006). Induction and specification of cranial placodes. Dev. Biol. 294, 303-351. Schlosser, G. and Ahrens, K. (2004). Molecular anatomy of placode development in Xenopus laevis. Dev. Biol. 271, 439-466. Semina, E. V., Brownell, I., Mintz-Hittner, H. A., Murray, J. C. and Jamrich, M. (2001). Mutations in the human forkhead transcription factor FOXE3 associated with anterior segment ocular dysgenesis and cataracts. Hum. Mol. Genet. 10, 231-236. Servetnick, M. and Grainger, R. M. (1991). Changes in neural and lens competence in Xenopus ectoderm: evidence for an autonomous developmental timer. Development 112, 177-188. Shi, X., Bosenko, D. V., Zinkevich, N. S., Foley, S., Hyde, D. R., Semina, E. V. and Vihtelic, T. S. (2005). Zebrafish pitx3 is necessary for normal lens and retinal development. Mech. Dev. 122, 513-527. Shi, X., Luo, Y., Howley, S., Dzialo, A., Foley, S., Hyde, D. R. and Vihtelic, T. S. (2006). Zebrafish foxe3: Roles in ocular lens morphogenesis through interaction with pitx3. Mech. Dev. 123, 761-782. Smith, A. N., Miller, L. A., Song, N., Taketo, M. M. and Lang, R. A. (2005). The duality of beta-catenin function: a requirement in lens morphogenesis and signaling suppression of lens fate in periocular ectoderm. Dev. Biol. 285, 477489. Smith, G. M. and Velzeboer, C. M. (1975). Peter’s anomaly. Ophthalmologica 171, 318-320. Soules, K. A. and Link, B. A. (2005). Morphogenesis of the anterior segment in the zebrafish eye. BMC Dev. Biol. 5, 12. Spemann, H. (1901). Ueber Korrelationen in der Entwicklung des Auges. Bonn: Jena Verlag. Spemann, H. (1912). Zur Entwicklung des Wirbeltierauges. Zool. Jahrb. Abt. Allg. Zool. Phys. Tiere 32, 1-98. DEVELOPMENT 1462 REVIEW Stone, L. S. (1953). An experimental analysis of lens regeneration. Am. J. Ophthalmol. 36, 31-39. Stone, L. S. (1967). An investigation recording all salamanders which can and cannot regenerate a lens from the dorsal iris. J. Exp. Zool. 164, 87-103. Swindell, E. C., Bailey, T. J., Loosli, F., Liu, C., Amaya-Manzanares, F., Mahon, K. A., Wittbrodt, J. and Jamrich, M. (2006). Rx-Cre, a tool for inactivation of gene expression in the developing retina. Genesis 44, 361-363. Ton, C. C., Hirvonen, H., Miwa, H., Weil, M. M., Monaghan, P., Jordan, T., van Heyningen, V., Hastie, N. D., Meijers-Heijboer, H., Drechsler, M. et al. (1991). Positional cloning and characterization of a paired box- and homeoboxcontaining gene from the aniridia region. Cell 67, 1059-1074. Torres, M. and Giraldez, F. (1998). The development of the vertebrate inner ear. Mech. Dev. 71, 5-21. Tsonis, P. A., Vergara, M. N., Spence, J. R., Madhavan, M., Kramer, E. L., Call, M. K., Santiago, W. G., Vallance, J. E., Robbins, D. J. and Del Rio-Tsonis, K. (2004). A novel role of the hedgehog pathway in lens regeneration. Dev. Biol. 267, 450-461. Valleix, S., Niel, F., Nedelec, B., Algros, M. P., Schwartz, C., Delbosc, B., Delpech, M. and Kantelip, B. (2006). Homozygous nonsense mutation in the FOXE3 gene as a cause of congenital primary aphakia in humans. Am. J. Hum. Genet. 79, 358-364. Vincent, A., Billingsley, G., Priston, M., Williams-Lyn, D., Sutherland, J., Glaser, T., Oliver, E., Walter, M. A., Heathcote, G., Levin, A. et al. (2001). Phenotypic heterogeneity of CYP1B1: mutations in a patient with Peters’ anomaly. J. Med. Genet. 38, 324-326. Vincent, A., Billingsley, G., Priston, M., Glaser, T., Oliver, E., Walter, M., Ritch, R., Levin, A. and Heon, E. (2006). Further support of the role of CYP1B1 in patients with Peters anomaly. Mol. Vis. 12, 506-510. Wawersik, S., Purcell, P., Rauchman, M., Dudley, A. T., Robertson, E. J. and Maas, R. (1999). BMP7 acts in murine lens placode development. Dev. Biol. 207, 176-188. Wigle, J. T., Chowdhury, K., Gruss, P. and Oliver, G. (1999). Prox1 function is crucial for mouse lens-fibre elongation. Nat. Genet. 21, 318-322. Williams, S. C., Altmann, C. R., Chow, R. L., Hemmati-Brivanlou, A. and Lang, R. A. (1998). A highly conserved lens transcriptional control element from the Pax-6 gene. Mech. Dev. 73, 225-229. REVIEW 1463 Wistow, G. and Piatigorsky, J. (1987). Recruitment of enzymes as lens structural proteins. Science 236, 1554-1556. Wolf, G. (1895). Entwicklungsphysiologische Studien. I. Die Regeneration der Urodelenlinse. Wilhelm Roux Arch. Entwickl. Mech. Org. 1, 380-390. Yamada, R., Mizutani-Koseki, Y., Hasegawa, T., Osumi, N., Koseki, H. and Takahashi, N. (2003). Cell-autonomous involvement of Mab21l1 is essential for lens placode development. Development 130, 1759-1770. Yang, Y., Stopka, T., Golestaneh, N., Wang, Y., Wu, K., Li, A., Chauhan, B. K., Gao, C. Y., Cveklova, K., Duncan, M. K. et al. (2006). Regulation of alphaAcrystallin via Pax6, c-Maf, CREB and a broad domain of lens-specific chromatin. EMBO J. 25, 2107-2118. Yoshida, K., Imaki, J., Koyama, Y., Harada, T., Shinmei, Y., Oishi, C., Matsushima-Hibiya, Y., Matsuda, A., Nishi, S., Matsuda, H. et al. (1997). Differential expression of maf-1 and maf-2 genes in the developing rat lens. Invest. Ophthalmol. Vis. Sci. 38, 2679-2683. Yoshimoto, A., Saigou, Y., Higashi, Y. and Kondoh, H. (2005). Regulation of ocular lens development by Smad-interacting protein 1 involving Foxe3 activation. Development 132, 4437-4448. Yu, J. K., Holland, L. Z., Jamrich, M., Blitz, I. L. and Hollan, N. D. (2002). AmphiFoxE4, an amphioxus winged helix/forkhead gene encoding a protein closely related to vertebrate thyroid transcription factor-2: expression during pharyngeal development. Evol. Dev. 4, 9-15. Zhang, L., Mathers, P. H. and Jamrich, M. (2000). Function of Rx, but not Pax6, is essential for the formation of retinal progenitor cells in mice. Genesis 28, 135142. Zhang, X., Friedman, A., Heaney, S., Purcell, P. and Maas, R. L. (2002). Meis homeoproteins directly regulate Pax6 during vertebrate lens morphogenesis. Genes Dev. 16, 2097-2107. Zilinski, C., Brownell, I., Hashimoto, R., Medina-Martinez, O., Swindell, E. and Jamrich, M. (2004). Expression of FoxE4 and Rx genes visualizes the timing and dynamics of critical processes taking place during initial stages of vertebrate eye development. Dev. Neurosci. 26, 1-14. Zilinski, C. A., Shah, R., Lane, M. E. and Jamrich, M. (2005). Modulation of zebrafish pitx3 expression in the primordia of the pituitary, lens, olfactory epithelium and cranial ganglia by hedgehog and nodal signaling. Genesis 41, 33-40. DEVELOPMENT Development 134 (8)