* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Lecture 1 - Imperial College London
Metabolomics wikipedia , lookup
Point mutation wikipedia , lookup
Metalloprotein wikipedia , lookup
Peptide synthesis wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Ribosomally synthesized and post-translationally modified peptides wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Genetic code wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
15-Hydroxyeicosatetraenoic acid wikipedia , lookup
Butyric acid wikipedia , lookup
Specialized pro-resolving mediators wikipedia , lookup
Citric acid cycle wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Biochemistry wikipedia , lookup
Biosynthesis of doxorubicin wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
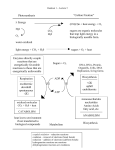
An Overview of Biosynthesis Pathways – Inspiration for Pharmaceutical and Agrochemical Discovery Alan C. Spivey [email protected] Oct 2016 Lessons in Synthesis - Azadirachtin • Azadirachtin is a potent insect anti-feedant from the Indian neem tree: – exact biogenesis unknown but certainly via steroid modification: O O MeO2C O O OAc H O OH C H 11 12 OH O 14 O 7 HO H OH AcO H AcO tirucallol (cf. lanosterol) – H OH H azadirachtanin A (a limanoid = tetra-nor-triterpenoid) oxidative cleavage of C ring 8 AcO MeO2C H OH O O OH highly hindered C-C bond for synthesis! O azadirachtin – – Intense synhtetic efforts by the groups of Nicolaou, Watanabe, Ley and others since structural elucidation in 1987. 1st total synthesis achieved in 2007 by Ley following 22 yrs of effort ~40 researchers and over 100 person-years of research! – 64-step synthesis – – Veitch Angew. Chem. Int. Ed. 2007, 46, 7629 (DOI) & Veitch Angew. Chem. Int. Ed. 2007, 46, 7633 (DOI) Review ‘The azadirachtin story’ see: Veitch Angew. Chem. Int. Ed. 2008, 47, 9402 (DOI) Format & Scope of Presentation • Metabolism & Biosynthesis – • Shikimate Metabolites – – • acetylCoA & the citric acid cycle → -amino acids → alkaloids Opioids – powerful pain killers Fatty Acids and Polyketides – – • photosynthesis & glycolysis → shikimate formation → shikimate metabolites Glyphosate – a non-selective herbicide Alkaloids – – • some definitions, 1° & 2° metabolites acetylCoA → malonylCoA → fatty acids, prostaglandins, polyketides, macrolide antibiotics NSAIDs – anti-inflammatory’s Isoprenoids/terpenes – – acetylCoA → mevalonate → isoprenoids, terpenoids, steroids, carotenoids Statins – cholesterol-lowering agents Metabolism and Biosynthesis Metabolism & Natural Product Diversity Me HO2C NMe H H O Me Me N N O O H N N H caffeine camphor HO H MeO N NH CO2 H2O Pi N2 hv lysergic acid N quinine Me O H OH O O N O Me O H CO2H N clavulanic acid Me N nicotine O O patulin OH androstenedione Metabolism • Metabolism is the term used for in vivo processes by which compounds are degraded, interconverted and synthesised: – Catabolic or degradative: primarily to release energy and provide building blocks • – Anabolic or biosynthetic: primarily to create new cellular materials (1° & 2° metabolites) • • generally oxidative processes/sequences (glycolysis, Krebs cycle) generally reductive processes/sequences These two types of process are coupled – one provides the driving force for the other: Natural product secondary metabolites CO 2 + H 2O complex metabolites 'CO 2 fixation' hv (photosynthesis) Nutrients energy storage O2 energy release Cell components & Growth ADP + P i Catabolism Energy & Building blocks ATP oxidative degredation NAD(P)H NAD(P) + H Anabolism reductive biosynthesis Building Blocks respiration 'nitrogen fixation' (by diazatrophs, lightening, the Haber process) simple products CO 2 + H 2O N2 Primary Metabolism - Overview Primary metabolites Primary metabolism Secondary metabolites CO2 + H2O PHOTOSY NTHESIS HO HOHO 1) 'light reactions': hv -> ATP and NADPH 2) 'dark reactions': CO2 -> sugars (Calvin cycle) oligosaccharides polysaccharides nucleic acids ( RNA, DNA) O HO OH glucose & other 4,5,6 & 7 carbon sugars glycolysis CO2 SHIKIMAT E METABOLIT ES cinnamic acid derivatives aromatic compounds lignans, f lavinoids PO CO2 PO phosphoenol pyruvate + HO HO O OH erythrose-4-phosphate OH OH shikimate aromatic amino acids CO2 aliphatic amino acids O pyruvate SCoA O acetyl coenzyme A CoAS tetrapyrroles (porphyrins) Citric acid cycle (Krebs cycle) SCoA CO2 O malonyl coenzyme A HO O O acetoacetyl coenzyme A peptides proteins ALKALOIDS penicillins cephalosporins cyclic peptides HO CO2 mevalonate saturated f atty acids unsaturated f atty acids lipids FATT Y ACIDS & POLY KETIDES prostaglandins polyacetylenes aromatic compounds, polyphenols macrolides ISOPRENOIDS terpenoids steroids carotenoids For interesting animations’ of e.g. photosynthesis see: http://www.johnkyrk.com/index.html Shikimate Metabolites Shikimate Metabolites H NH3 O2C NH3 H NH3 CO2 NH CO2 H n Me O (S)-tryptophan (ArC0) O O (S)-phenylalanine (ArC3) OH (S)-tyrosine (ArC3) O MeO SHIKIMATE METABOLITES menaquinone (vitamin K2) (ArC1) OH OH H H HO OH O H 3 O scopoletin (ArC3) O O O Me Me H H MeO OH -tocopherol (vitamin E) (ArC1) O OH OMe OMe HO OH OH epigallocatechin (EGC) (ArC3) podophyllotoxin (ArC3) The Shikimate Biosynthetic Pathway - Overview • Phosphoenol pyruvate & erythrose-4-phosphate → shikimate → chorismate → prephenate: – The detailed mechanisms of these steps have been studied intensively. Most are chemically complex and interesting. For additional details see: • • • Mann Chemical Aspects of Biosynthesis Oxford Chemistry Primer No. 20, 1994 (key details) Haslam Shikimic Acid – Metabolism and Metabolites Wiley, 1993 (full details and primary Lit. citations) http://www.chem.qmul.ac.uk/iubmb/enzyme/reaction/misc/shikim.html (interesting web-site with many biosynethtic pathways) Rational Agrochemical Development – Shikimate Pathway Intervention • The shikimate biosynthetic pathway is not found in animals/humans – only in plants – • selective intervention in these pathways allows development of agrochemicals with minimal human toxicity Glyphosate (‘Roundup’) – a Monsanto agrochemical is a potent inhibitor of the conversion of 3-phosphoshikimate (3-PS) → 5-enolpyruvylshikimate-3-phosphate (5-EPS-3P) – a non-selective herbicide H NH3 CO2 CO2 CO2 PO CO2 phosphoenol pyruvate ( PEP) PO HO CO2 PEP PO + OH PO OH O CO2 O OH H OP Pi 3-phosphoshikimate ( 3-PS) O CO2 glyphosate ( Roundup® ) inhibits this step PO N H2 CO2 OH ( S)-tyrosine OH 5-enolpyruvylshikimate-3-phosphate ( 5-EPS-3P) OH erythrose-4-phosphate ( E-4-P) PO H NH3 CO2 ( S)-phenylalanine Chorismate → Tryptophan, Tyrosine & Phenylalanine • Chorismate → anthranilate → tryptophan ribose derived CO2 O OH • O2C CO2 'NH3' NH2 NH3 NH CO2 chorismate (S)-tryptophan [78 ATP equivs] ArC 0 anthranilate Chorismate → prephenate → tyrosine & phenylalanine – NB. The enzyme chorismate mutase [EC 5.4.99.5] which mediates the conversion of chorismate to prephenate is the only known ‘Claisen rearrangementase’ H NH3 CO2 CO2 Claisen rearangement CO2 NAD H3N H OH CO2 chorismate chorismate mutase [EC 5.4.99.5] 'NH3' OH prephenate ArC3 OH O O (S)-tyrosine [62 ATP equivs] CO2 O2C O2C CO2 NADH H NH3 OH (S)-arogenate H2O CO2 CO2 (S)-phenylalanine [65 ATP equivs] ArC3 Tyrosine/Phenylalanine → ArC3 Metabolites • Tyrosine & phenylalanine → cinnamate derivatives → ArC3 metabolites – coumarins, lignans (stereoselective enzymatic dimerisation) & lignins (stereorandom radical polymerisation) O O scopoletin (a coumarin) germination stimulant MeO ArC3 OH OH H H NH2 H CO2 phenylalanine ammonia lyase (PAL) CO2 CO2 OH O H O O O H and O H X X = OH X=H X cinnamate derivatives H OMe MeO MeO HO OMe podophyllotoxin (a lignan) natural product used to treat worts OH ferulate OMe H MeO NH4 H O pinoresinol (a lignan) 2 x ArC3 OMe HO HO O O OMe O fragment of lignin polymer 'woody' component of cell walls O O MeO OH OH OMe OH n x ArC3 Primary Metabolism - Overview Primary metabolites Primary metabolism Secondary metabolites CO2 + H2O PHOTOSY NTHESIS HO HOHO 1) 'light reactions': hv -> ATP and NADPH 2) 'dark reactions': CO2 -> sugars (Calvin cycle) oligosaccharides polysaccharides nucleic acids ( RNA, DNA) O HO OH glucose & other 4,5,6 & 7 carbon sugars glycolysis CO2 SHIKIMAT E METABOLIT ES cinnamic acid derivatives aromatic compounds lignans, f lavinoids PO CO2 PO phosphoenol pyruvate + HO HO O OH erythrose-4-phosphate OH OH shikimate aromatic amino acids CO2 aliphatic amino acids O pyruvate SCoA O acetyl coenzyme A CoAS tetrapyrroles (porphyrins) Citric acid cycle (Krebs cycle) SCoA CO2 O malonyl coenzyme A HO O O acetoacetyl coenzyme A peptides proteins ALKALOIDS penicillins cephalosporins cyclic peptides HO CO2 mevalonate saturated f atty acids unsaturated f atty acids lipids FATT Y ACIDS & POLY KETIDES prostaglandins polyacetylenes aromatic compounds, polyphenols macrolides ISOPRENOIDS terpenoids steroids carotenoids For interesting animations’ of e.g. photosynthesis see: http://www.johnkyrk.com/index.html Alkaloids Alkaloids • Definitions: – – originally – ‘a natural product that could be extracted out of alkaline but not acidic water’ (i.e. containing a basic amine function that protonated in acid) more generally - ‘any non-peptidic & non-nucleotide nitrogenous secondary metabolite’ H OH O N O H N H coniine N HO Me N nicotine OH H N CO2H H N retronecine clavulanic acid N H H HO MeO H ALKALOIDS H N sparteine HO N quinine HO2C N H O NMe H H N O H H H NMe HO morphine H H O strychnine NH lysergic acid The Citric Acid Cycle • The citric acid (Krebs) cycle is a major catabolic pathway of 1° metabolism that provides two key building blocks for aliphatic amino acid biosynthesis - oxaloacetate & -ketoglutarate: CO2 NADH CO2 SCoA O CoA O acetyl coenzyme A pyruvate OVERAL STOICHIOMETRY NAD CO2 1x CO2 CO2 NADH CO2 OH NAD HO CO2 O CO2 CO2 CO2 oxaloacetate O 'acetate' CO2 H2O CO2 cis-aconitate citrate HO + CO2 H2O CO2 1x O2 CO2 CO2 malate isocitrate aliphatic amino acids NAD H2O CO2 CO2 CoA CO2 NADH GTP FADH2 CO2 fumarate O O SCoA CO2 O O CO2 NADH CO2 CO2 2x FAD oxalosuccinate CO2 succinate Pi + GDP CO2 CO2 CO2 succinyl-SCoA CoA NAD + CO2 -ketoglutarate THE CITRIC ACID CYCLE 12x ATP energy! The Biosynthesis of Lysine & Ornithine • Lysine & ornithine - the two most significant, non-aromatic -amino acid precursors to alkaloids: – – – NB. lysine (Lys) is proteinogenic whereas ornithine (Orn) is not phenylalanine (Phe), tyrosine (Tyr) & tryptophan (Trp) from shikimate are the other important precursors biosynthesis is via reductive amination of the appropriate -ketoacid mediated by pyridoxal-5’-phosphate (PLP) O O R NH2 reductive amination NH3 OH R O O ketoacid O R O amino acid NH3 O H3N NH3 O O P O O pyridoxamine phosphate O N H tightly bound to enzyme O O P O O Me O CHO O N H pyridoxal phosphate lysine (Lys) [50 ATP equivs] Me NH3 O H3N NH3 O O citric acid cycle O O O O -ketoglutarate oxidative deamination OVERALL: TRANSAMINATION O O O glutamic acid (Glu) O ornithine (Orn) [<44 ATP equivs (=Arg)] PLP Chemistry – Transamination & Racemisation • Transamination – LHS → RHS (reductive amination); RHS → LHS (oxidative deamination): Enz-NH2 OP NH3 O N H pyridoxamine phosphate R CO2 O R CO2 N H OP H Enz-NH3 R CO2 N OP O H2O imine formation N H H O N H transaminase Enz-NH2 H R CO2 N OP N H H O Enz imine OP exchange R H CO2 NH3 N H O N H pyridoxal phosphate bound as imine PLP Chemistry – Decarboxylation • Decarboxylation: OP Enz N H O R H Enz-NH2 CO2 H O R NH3 OP imine exchange N H pyridoxal phosphate bound as imine R O N Enz-NH2 Enz-NH3 H O N OP H R H O H N OP H R N H N H NH3 decarboxylase • Enz OP O CO2 N H imine exchange N H O N H pyridoxal phosphate bound as imine Decarboxylation of lysine & ornithine: PLP dependant decarboxylase HO2C NH2 NH2 CO2 PLP H2N lysine NH2 RHN NH2 NH2 NH2 ornithine O cadaverine PLP dependant decarboxylase HO2C RHN PLP H2N CO2 N R iminium salt NH2 putrescine RHN NH2 RHN O N R iminium salt piperidine alkaloids pyrrolidine alkaloids Lysine-derived Piperidine Alkaloids – Hemlock! Socrates drinking poison hemlock, 399 B.C. "The Death of Socrates" by Jacques-Louis David (1787) Piperidine Alkaloids – Pelletierine & Coniine • Pelletierine: lysine O hydrolysis O N R from lysine • O N R SCoA O acetoacetylCoA SCoA O N R CO2 R = H pelletrine R = Me, N-methylpelletrine Coniine: – in 399 BC Socrates was sentenced to death for impiety and executed by being forced to drink a potion made from poison hemlock. The toxic component in hemlock is coniine. Although by analogy with the above pathway, biosynthesis from lysine might be suspected, it is in fact of fatty acid origin 3x O O SCoA CO2 malonylCoA O SCoA O [O] O SCoA SCoA acetylCoA NADPH fatty acid NADPH O N H H coniine NH2 PLP reductive amination N NADP coniceine H2O O NH3 CO2 CO2 NADP O O Tyrosine-derived Alkaloids - Opium Alkaloids Benzylisoquinoline Alkaloids papaverine morphine Benzylisoquinoline Alkaloids – Ring Formation • Benzylisoquinoline alkaloids constitute an extremely large and varied group of alkaloids – many, particularly the opium alkaloids (e.g. papaverine, morphine) are biosynthesised from two molecules of tyrosine via nor-laudanosoline: – Mechanism of Pictet Spengler reaction: HO DHPP + dopamine HO HO HO H NH HO2C HO OH OH HO2C NH NH HO OH OH HO2C OH OH nor-laudanosoline-1-carboxylic acid Benzylisoquinoline Alkaloids - Papaverine • Papaverine: analgesic contsituent of the opium poppy (Papaver somniferum): – biosynthesis: – NB. The prefix nor means without a methyl group. Laudanosoline, reticuline and laudanosine are the N-methyl compounds Oxidative Phenolic Coupling – Morphine & Synthetic Opioids • Morphine: analgesic & sedative contsituent of the opium poppy (Papaver somniferum): – biosynthesis: o-/p- oxidative phenolic coupling of reticuline: – – Morphine acts by activating the opiate receptors in the brain (IC50 3 nM) The natural ligands for these receptors are peptides: e.g. Leu-enkephalin (Tyr–Gly–Gly–Phe–Leu) (IC50 12 nM) Dimeric Indole Alkaloids – Vinca extracts Dimeric Indole Alkaloids vinblastine (R = Me) vincristine (R = CHO) Potent anti tumour alkaloids used in cancer chemotherapy Tryptamine + Secolaganin → Strictosidine • Most alkaloids of mixed Tryptophan/mevalonate biogenesis (>1200) are derived from strictosidine: – Strictosidine is derived from the condensation of tryptamine with the iridoid C10 monoterpene secologanin: OPP O H HO CO2 HO H O MeO2C mevalonate (x2) geranyl pyrophosphate see isoprenoids OGlu tryptophan enzymatic Pictet-Spengler reaction secologanin CO2 NH3 N H H H H2O PLP NH2 MeO2C OGlu isoprene O strictosidine N H CO2 tryptophan – NH N H H tryptamine Mechanism of Pictet-Spengler reaction: • via spirocyclic intermediate then Wagner-Meerwein 1,2-alkyl shift: NH2 N H H + H MeO2C tryptamine N H O NH H OGlu O secologanin H MeO2C O OGlu N H H MeO2C NH NH N Wagner-Meerwein H H H OGlu 1,2-alkyl shift H H O spirocyclic intermediate MeO2C O N H H NH H OGlu H MeO2C O strictosidine OGlu Strictosidine → Vinca, Strychnos, Quinine etc. • The diversity of alkaloids derived from strictosidine is stunning and many pathways remain to be fully elucidated: N OH N H N H MeO2C H H H O MeO2C ajmalicine (vinca) OAc N H OH Me CO2Me MeO N N H H H H MeO2C OH vinblastine (vinca) MeO N N H H yohimbine O N N N N H H O OH O 3 NH H H H MeO2C camptothecin H N H aspidospermine (vinca) OGlu O strictosidine (isovincoside) N H HO H MeO N Me N H N O N quinine H O N O H gelsemine (oxindole) H H H O strychnine (strychnos) Primary Metabolism - Overview Primary metabolites Primary metabolism Secondary metabolites CO2 + H2O PHOTOSY NTHESIS HO HOHO 1) 'light reactions': hv -> ATP and NADPH 2) 'dark reactions': CO2 -> sugars (Calvin cycle) oligosaccharides polysaccharides nucleic acids ( RNA, DNA) O HO OH glucose & other 4,5,6 & 7 carbon sugars glycolysis CO2 SHIKIMAT E METABOLIT ES cinnamic acid derivatives aromatic compounds lignans, f lavinoids PO CO2 PO phosphoenol pyruvate + HO HO O OH erythrose-4-phosphate OH OH shikimate aromatic amino acids CO2 aliphatic amino acids O pyruvate SCoA O acetyl coenzyme A CoAS tetrapyrroles (porphyrins) Citric acid cycle (Krebs cycle) SCoA CO2 O malonyl coenzyme A HO O O acetoacetyl coenzyme A peptides proteins ALKALOIDS penicillins cephalosporins cyclic peptides HO CO2 mevalonate saturated f atty acids unsaturated f atty acids lipids FATT Y ACIDS & POLY KETIDES prostaglandins polyacetylenes aromatic compounds, polyphenols macrolides ISOPRENOIDS terpenoids steroids carotenoids For interesting animations’ of e.g. photosynthesis see: http://www.johnkyrk.com/index.html Fatty Acids Fatty Acid Primary Metabolites • OH OH glycerol Primary metabolites: – fully saturated, linear carboxylic acids & derived (poly)unsaturated derivatives: • • • • OH constituents of essential natural waxes, seed oils, glycerides (fats) & phospholipids structural role – glycerides & phospholipids are essential constituents of cell membranes energy storage – glycerides (fats) can also be catabolised into acetate → citric acid cycle biosynthetic precursors – for elaboration to secondary metabolites 3x fatty acids OCOR1 OCOR2 OCOR3 glycerides SATURATED ACIDS [MeCH2(CH2CH2)nCH2CO2H (n = 2-8)] e.g. CO2H 8 CO2H capric acid (C8, n = 3) 1 CO2H 1 16 CO2H lauric acid (C12, n = 4) 1 12 1 14 CO2H 10 CO2H caprylic acid (C8, n = 2) 1 1 18 MONO-UNSATURATED ACID DERIVATIVES (MUFAs) e.g. myristic acid (C14, n = 5) palmitic acid (C16, n = 6) stearic acid (C18, n = 7) 9 9 CO2H CO2H 1 1 palmitoleic acid (C16, Z -9) 16 oleic acid (C18, Z-9) 18 (>80% of fat in olive oil ) POLY-UNSATURATED ACID DERIVATIVES (PUFAs) e.g. 8 5 CO2H 1 20 11 14 8 5 CO2H arachidonic acid (AA) 1 (C20, Z -5, Z -8, Z -11, Z -14) 11 14 17 eicosapentaenoic acid (EPA) (C20, Z-5, Z -8, Z-11, Z -14, Z -17) 20 (in cod liver oil) Fatty Acids Derivatives – Secondary Metabolites • Secondary metabolites – further elaborated derivatives of polyunsaturated fatty acids (PUFAs) • e.g. polyacetylenes & ‘eicosanoids’ (prostaglandins, thromboxanes & leukotrienes) Biosynthesis of Fatty Acids – Iterative Oligomerisation • fatty acids are biosynthesised from acetyl CoA as a starter unit by iterative ‘head-to-tail’ oligomerisation involving: – – • condensation with malonyl CoA as an extender unit (with loss of CO2) – a decarboxylative Claisen condensation 3-step reduction of the resulting ketone → methylene after n = 2-8 iterations the C8-20 saturated fatty acid is released from the enzyme(s): Biosynthesis of Fatty Acids – Overview of FAS • The in vivo process by which all this takes place involves a ‘molecular machine’ - Fatty Acid Synthase (FAS) – – – Type I FAS: single multifunctional protein complex (e.g. in mammals incl. humans) Type II FAS: set of discrete, dissociable single-function proteins (e.g. in bacteria) All FASs comprise 8 components (ACP & 7× catalytic activities): ACP, KS, AT, MT, KR, DH, ER & [TE] : O O O O S AT CoAS O O MT SH KS S S O decarboxylative Claisen condensation SH S reduction SH 1) KR 2) DH 3) ER n = 2-8 cycles ACP n O S SH O S SH SH S translocation O TE OH O KS ACP KS ACP KS ACP CoAS n SH O O O KS ACP KS ACP KS ACP KS = keto synthase (also known as CE = condensing enzyme); AT = acetyl transferase; MT = malonyl transferase; KR = keto reductase; DH = dehydratase; ER = enoyl reductase; TE = thioesterase; ACP = acyl carrier protein O Human Fatty Acid Synthase (FAS) • the first three-dimensional structure of human fatty acid synthase (272 kDa) at 4.5 Å resolution by Xray crystallography: – Maier, Jenni & Ban Science 2006, 311, 1258 (DOI) ; also Fungal FAS @ 3.1 Å resolution see: Jenni et al. Science 2007, 316, 254 & 288 Structural overview. (A) Front view: FAS consists of a lower part comprising the KS (lower body) and MAT domains (legs) connected at the waist with an upper part formed by the DH, ER (upper body), and KR domains (arms). (B) Top view of FAS with the ER and KR domains resting on the DH domains. (C) Bottom view showing the arrangement of the KS and MAT domains and the continuous electron density between the KS and MAT domains FATTY ACID BIOSYNTHESIS (type II FAS) ACP1 AT1 KS1 Cys KR1 DH1 ER1 ACP2 MT2 SH Pantetheine SH NB. the following sequence of slides have been adapted from: http://www.courses.fas.harvard.edu/%7echem27/ SH FATTY ACID BIOSYNTHESIS ACP1 AT1 KS1 Cys KR1 DH1 ER1 ACP2 MT2 SH Pantetheine O SH Me S Co Acetyl-CoA • AT1 loads acetyl group onto KS1 SH FATTY ACID BIOSYNTHESIS ACP1 AT1 KS1 Cys O Pantetheine SH KR1 DH1 ER1 ACP2 MT2 S Me SH FATTY ACID BIOSYNTHESIS ACP1 AT1 KS1 Cys O Pantetheine KR1 DH1 ER1 ACP2 S Me SH O -O O S MT2 Co Malonyl-CoA • AT1 loads malonyl group onto ACP1 SH FATTY ACID BIOSYNTHESIS ACP1 AT1 KS1 Cys O Pantetheine S O DH1 ER1 ACP2 MT2 S Me SH O O KR1 - FATTY ACID BIOSYNTHESIS ACP1 AT1 KS1 Cys O Pantetheine KR1 DH1 ER1 ACP2 S Me CO2 S O O O MT2 - • KS1 catalyzes Claisen condensation SH FATTY ACID BIOSYNTHESIS ACP1 AT1 KS1 Cys KR1 DH1 ER1 ACP2 MT2 SH Pantetheine S O O Me SH FATTY ACID BIOSYNTHESIS ACP1 AT1 KS1 Cys Pantetheine SH KR1 S DH1 ER1 ACP2 MT2 Me O O SH • KR1 catalyzes reduction of ketone FATTY ACID BIOSYNTHESIS ACP1 AT1 KS1 Cys Pantetheine SH KR1 S DH1 ER1 ACP2 MT2 Me O OH SH FATTY ACID BIOSYNTHESIS ACP1 AT1 KS1 Cys Pantetheine SH KR1 DH1 S ER1 ACP2 MT2 Me O OH SH • DH1 catalyzes dehydration of alcohol FATTY ACID BIOSYNTHESIS ACP1 AT1 KS1 Cys Pantetheine SH KR1 DH1 S ER1 ACP2 MT2 Me O SH FATTY ACID BIOSYNTHESIS ACP1 AT1 KS1 Cys Pantetheine SH KR1 DH1 ER1 S ACP2 MT2 Me O SH • ER1 catalyzes reduction of alkene FATTY ACID BIOSYNTHESIS ACP1 AT1 KS1 Cys Pantetheine SH KR1 DH1 ER1 S ACP2 MT2 Me O SH FATTY ACID BIOSYNTHESIS ACP1 AT1 KS1 Cys Pantetheine KR1 DH1 ER1 ACP2 MT2 KS2 Cys SH S O Me SH • KS2 catalyzes translocation to module 2 SH KR H1 FATTY ACID BIOSYNTHESIS ER1 ACP2 MT2 KS2 Cys Pantetheine S O Me SH KR2 DH2 ER2 TE Ser OH H1 FATTY ACID BIOSYNTHESIS ER1 ACP2 MT2 KS2 Cys KR2 DH2 ER2 TE S O Pantetheine Me SH O -O O S Co Malonyl-CoA • MT2 loads malonyl group onto ACP2 Ser OH H1 FATTY ACID BIOSYNTHESIS ER1 ACP2 MT2 KS2 Cys S O Pantetheine Me S O O O - KR2 DH2 ER2 TE Ser OH H1 FATTY ACID BIOSYNTHESIS ER1 ACP2 MT2 KS2 Cys KR2 DH2 ER2 TE S O Pantetheine Me S CO2 O O O - • KS2 catalyzes Claisen condensation Ser OH H1 FATTY ACID BIOSYNTHESIS ER1 ACP2 MT2 KS2 Cys Pantetheine S O O Me SH KR2 DH2 ER2 TE Ser OH H1 FATTY ACID BIOSYNTHESIS ER1 ACP2 MT2 KS2 Cys Pantetheine SH KR2 DH2 ER2 TE Ser S Me O O • KR2 catalyzes reduction of ketone OH H1 FATTY ACID BIOSYNTHESIS ER1 ACP2 MT2 KS2 Cys Pantetheine SH KR2 DH2 ER2 TE Ser S Me O OH OH H1 FATTY ACID BIOSYNTHESIS ER1 ACP2 MT2 KS2 Cys Pantetheine SH KR2 DH2 S ER2 TE Me O OH • DH2 catalyzes dehydration of alcohol Ser OH H1 FATTY ACID BIOSYNTHESIS ER1 ACP2 MT2 KS2 Cys Pantetheine SH KR2 DH2 S ER2 Me O TE Ser OH H1 FATTY ACID BIOSYNTHESIS ER1 ACP2 MT2 KS2 Cys Pantetheine SH KR2 DH2 TE ER2 S Me O • ER2 catalyzes reduction of alkene Ser OH H1 FATTY ACID BIOSYNTHESIS ER1 ACP2 MT2 KS2 Cys Pantetheine SH KR2 DH2 ER2 S TE Me O Ser OH H1 FATTY ACID BIOSYNTHESIS ER1 ACP2 MT2 KS2 Cys Pantetheine KR2 DH2 TE ER2 Ser SH S O Me • TE catalyzes transesterification OH H1 FATTY ACID BIOSYNTHESIS ER1 ACP2 MT2 KS2 Cys KR2 DH2 ER2 TE SH O O Pantetheine SH Me Ser H1 FATTY ACID BIOSYNTHESIS ER1 ACP2 MT2 KS2 Cys KR2 DH2 ER2 TE SH O O Pantetheine SH Me • TE catalyzes hydrolysis Ser H OH H1 FATTY ACID BIOSYNTHESIS ER1 ACP2 MT2 KS2 Cys KR2 DH2 ER2 TE Ser SH OH Pantetheine SH OH O Me Biosynthesis of Unsaturated Fatty Acids • two mechanisms are known for the introduction of double bonds into fatty acids: – – in BACTERIA: anaerobic [O] → monounsaturated FAs (MUFAs) in MAMMALS, INSECTS & PLANTS: aerobic [O] → MUFAs & polyunsaturated FAs (PUFAs) Rational Anti-inflammatory Development – Prostaglandin & Thromboxane Pathway Intervention • • prostaglandins & thromboxanes are derived from further oxidative processing of arachiodonic acid both are important hormones which control e.g. smooth muscle contractility (blood pressure), gastric secretion, platelet aggregation & inflammation (<nM activity) – various pharmaceuticals including corticosteroids & asprin inhibit biosynthethetic steps in these pathways Polyketides Polyketides • the structural variety of polyketide secondary metabolites is very wide: – NB. starter units marked in red; extender units in bold black; post oligomerisation appended groups in blue Me HO O CO2H OH 6-methylsalicylic acid (antibiotic) OMe O OH O CO2H OH Me orsellinic acid O CO2H 2 H O MeO OH citrinin (kidney toxin 'yellow rice disease') O Cl Griseofulvin (treatment for ring worm infections) O O O O OH MeO O O O H O POLYKETIDES OH HO O OH OH OH O rapamycin (immunosuppressant) NB. a mixed polypropionate/acetate aflatoxin B1 (mycotoxic carcingen) O O O MeO OH H O H O MeO O N CO2H OH O actinorhodin (antibiotic) OMe HO O OH 6-deoxyerythronolide B NB. a polypropionate HO O O OH O erythromycin A (antibiotic) NMe2 O O Me O O Me O O OMe OH mevinolin (=lovastatin®) (anti-cholesterol) O Biosynthesis of Polyketides – Oligomerisation Steps • polyketides are biosynthesised by a process very similar to that for fatty acids – the key differences are: • • – greater variety of starter units, extender units & termination processes absent or incomplete reduction of the iteratively introduced -carbonyl groups: ie. each cycle may differ in terms of KR, DH & ER modules & stereochemistry this leads to enormous diversity... Biosynthesis of Polyketides – Overview of PKS • the in vivo process of polyketide synthesis involves PolyKetide Synthases (PKSs): – PKSs (except Type II, see later) comprise the same 8 components as FASs. i.e. (ACP & 7× catalytic activities): ACP, KS, AT, MT, [KR, DH, ER & TE] Type I PKSs: single (or small set of) multifunctional protein complex(es) – • • – modular (microbial) - each ‘step’ has a dedicated catalytic site (→ macrolides) iterative (fungal) – single set of catalytic sites, each of which may operate in each iteration (cf. FASs) (→ aromatics/polyphenols - generally) Type II PKSs: single set of discrete, dissociable single-function proteins • iterative (microbial) - each catalytic module may operate in each iteration (cf. FASs) (→ aromatics/polyphenols) O Type I (modular & iterative): [Type II see later] O O O S SH AT SH MT KS S O decarboxylative Claisen condensation SH S 1) ± KR 2) ± DH 3) ± ER n cycles ACP O O n O S SH O S SH SH S translocation O TE OH O KS ACP KS ACP SH O n S reduction ? O O O O KS ACP CoAS O CoAS O O O CE ACP KS ACP KS = keto synthase; AT = acetyl transferase; MT = malonyl transferase; KR = keto reductase; DH = dehydratase; ER = enoyl reductase; TE = thioesterase; ACP = acyl carrier protein KS ACP O POLYKETIDE BIOSYNTHESIS [Type I – (modular)] ACP0 AT0 ACP1 AT1 KS1 Cys Pantetheine DH1 ER1 ACP2 SH Pantetheine Pantetheine SH KR1 SH NB. the following sequence of slides has also been adapted from: http://www.courses.fas.harvard.edu/%7echem27/ A POLYKETIDE BIOSYNTHESIS ACP0 AT0 ACP1 AT1 KS1 Cys Pantetheine KR1 DH1 ER1 SH Pantetheine Pantetheine SH Me SH O S ACP2 Co Propionyl-CoA • AT0 loads starting group (propionyl) onto ACP0 A POLYKETIDE BIOSYNTHESIS ACP0 AT0 ACP1 AT1 KS1 Cys Pantetheine O Me DH1 ER1 ACP2 SH Pantetheine Pantetheine S KR1 SH A POLYKETIDE BIOSYNTHESIS ACP0 AT0 ACP1 AT1 KS1 Cys Pantetheine Pantetheine S O KR1 DH1 ER1 ACP2 SH Pantetheine Me SH • KS1 catalyzes translocation to module 1 A POLYKETIDE BIOSYNTHESIS ACP0 AT0 ACP1 AT1 KS1 Cys Pantetheine SH ER1 ACP2 Pantetheine Me SH DH1 S O Pantetheine KR1 A POLYKETIDE BIOSYNTHESIS P0 AT0 ACP1 AT1 KS1 Cys KR1 DH1 ER1 ACP2 AT2 S O Pantetheine Me SH O SH -O O Me S Co Methylmalonyl-CoA • AT1 loads methylmalonyl group onto ACP1 SH P0 POLYKETIDE BIOSYNTHESIS AT0 ACP1 AT1 KS1 Cys KR1 DH1 ER1 ACP2 AT2 S O Pantetheine Me S O SH O SH Me O - P0 POLYKETIDE BIOSYNTHESIS AT0 ACP1 AT1 KS1 Cys KR1 DH1 ER1 ACP2 AT2 S O Pantetheine Me CO2 S O SH O Me O - • KS1 catalyzes Claisen condensation SH P0 POLYKETIDE BIOSYNTHESIS AT0 ACP1 AT1 KS1 Cys KR1 DH1 ER1 ACP2 AT2 SH Pantetheine S O SH SH * Me O Me Stereocenter P0 POLYKETIDE BIOSYNTHESIS AT0 ACP1 AT1 KS1 Cys Pantetheine SH KR1 DH1 ER1 ACP2 AT2 Me * S O O Me SH SH • KR1 catalyzes reduction of ketone P0 POLYKETIDE BIOSYNTHESIS AT0 ACP1 AT1 KS1 Cys Pantetheine SH KR1 DH1 ACP2 AT2 Me * S O * Me OH Stereocenter SH ER1 SH P0 POLYKETIDE BIOSYNTHESIS AT0 ACP1 AT1 KS1 Cys KR1 SH Pantetheine DH1 ER1 ACP2 AT2 Me * S O * Me OH SH SH • no DH1 activity P0 POLYKETIDE BIOSYNTHESIS AT0 ACP1 AT1 KS1 Cys KR1 SH Pantetheine DH1 ER1 ACP2 AT2 Me S * O * Me OH SH SH • no ER1 activity POLYKETIDE BIOSYNTHESIS ACP1 AT1 KS1 Cys KR1 DH1 ER1 ACP2 AT2 KS2 Cys SH S O Pantetheine HO * * Me Me SH • KS2 catalyzes translocation to module 2 SH KR H1 POLYKETIDE BIOSYNTHESIS ER1 ACP2 AT2 KS2 Cys Pantetheine O HO * Me SH DH2 ER2 TE Ser S * KR2 OH Me Biosynthesis of Erythromycin – Type I(modular) PKS • 6-deoxyerthronolide is a precursor to erythromycin A – bacterial antibiotic (Streptomyces erythreus): – – propionate based heptaketide; 3 multifunctional polypeptides (DEBS1, DEBS2 & DEBS3, all ~350 kDa) Katz et al. Science 1991, 252, 675 (DOI); Staunton, Leadley et al. Science 1995, 268, 1487 (DOI); Khosla et al. J. Am. Chem. Soc. 1995, 9105 (DOI); review: Staunton & Weissman Nat. Prod. Rep. 2001, 18, 380 (DOI) DEBS1 module 1 DEBS2 module 2 module 3 DEBS3 module 4 module 5 module 6 O KR AT KS AT DH ER KR KS AT S S O KR KS AT S KS AT KR KR KS AT S S O KS AT S S O O O HO HO O HO HO O HO HO O HO HO TE O O HO HO OH release O OH O OH HO loading O O OH HO HO HO OH O O OH OH = Acyl Carrier Protein erythronolide B TE = Thioesterase Type II PKSs – Enzyme Clusters (Microbial) • Type II PKSs: single set of discrete, dissociable single-function proteins (ACP & 6× catalytic functions): ACP, KS, KS, [KR, DH, ER, & TE] [NB. NO acetyl or malonyl transferases (AT, MT)] – • • iterative - each catalytic module may operate in each iteration (cf. FASs) (→ aromatics/polyphenols) these clusters (generally) use malonate as BOTH starter & extender unit their ACP proteins are able to load malonate direct from malonyl CoA (no MT required) the starter malonate is decarboxylated by ‘ketosynthase’ (KS) to give S-acetyl-ACP the extender malonates undergo decarboxylative Claisen condensations by ketosynthase (KS) – – • these clusters rarely utilise KR, DH or ER activities and produce ‘true’ polyketides: O CONH2 Type II (iterative): O S O SH O S O O S SH CoAS O O O O S S O CoAS KS ACP KS ACP O KS ACP S O KS ACP O n O 1) ± KR 2) ± DH 3) ± ER S SH O O S SH SH S translocation O TE OH O n cycles O n SH reduction (rarely) SH O decarboxylative Claisen condensation KS ACP KS ACP CONH2 O O O KS ACP KS ACP KS ACP KS = 'keto synthase ' (=decarboxylase!); KS = 'keto synthase ' (=ketosynthase!); KR = keto reductase; DH = dehydratase; ER = enoyl reductase; TE = thioesterase; ACP = acyl carrier protein O Biosynthesis of Actinorhodin – Type II PKS • actinorhodin – octaketide bacterial antibiotic (Streptomyces coelicolor) – Hopwood Chem. Rev. 1997, 97, 2465 (DOI) 7x O O CoAS O CoAS O O PKS [ACP, KS , KS] O O O O O O O O O 1 8x CO2 octaketide O O 16 cyclase O O O O O 16 SEnz O O 16 O 1 OH KR SEnz O HO O O SEnz 1 OH aromatase OH OH O O O OH 16 2 H OH O actinorhodin – O O octaketide synthesis then cyclisation? (as shown above) hexaketide synthesis then cyclisation then two further rounds of extension? indications can sometimes be gleaned from biomimetic syntheses... 16 O CO2H OH O cyclase 1 SEnz timing of 1st cyclisation and mechanism of control of chain length uncertain • • – O O O O 1 SEnz Scope of Structures - Type II PKS • microbial polyphenolic metabolites: pentaketides (5x C2) OMe O OH O O OH octaketides (8x C2) emodin eugenone MeO HO OMe O hexaketides (6x C2) nonaketides (9x C2) OH O OH O O O NH2 tetracycline plumbagin Me OH heptaketides (7x C2) H OH H O MeO O O OH NMe2 O OH decaketides (10x C2) rabelomycine rubrofusarin OH • OH O many display interesting biological activities... OH O OH Primary Metabolism - Overview Primary metabolites Primary metabolism Secondary metabolites CO2 + H2O PHOTOSY NTHESIS HO HOHO 1) 'light reactions': hv -> ATP and NADPH 2) 'dark reactions': CO2 -> sugars (Calvin cycle) oligosaccharides polysaccharides nucleic acids ( RNA, DNA) O HO OH glucose & other 4,5,6 & 7 carbon sugars glycolysis CO2 SHIKIMAT E METABOLIT ES cinnamic acid derivatives aromatic compounds lignans, f lavinoids PO CO2 PO phosphoenol pyruvate + HO HO O OH erythrose-4-phosphate OH OH shikimate aromatic amino acids CO2 aliphatic amino acids O pyruvate SCoA O acetyl coenzyme A CoAS tetrapyrroles (porphyrins) Citric acid cycle (Krebs cycle) SCoA CO2 O malonyl coenzyme A HO O O acetoacetyl coenzyme A peptides proteins ALKALOIDS penicillins cephalosporins cyclic peptides HO CO2 mevalonate saturated f atty acids unsaturated f atty acids lipids FATT Y ACIDS & POLY KETIDES prostaglandins polyacetylenes aromatic compounds, polyphenols macrolides ISOPRENOIDS terpenoids steroids carotenoids For interesting animations’ of e.g. photosynthesis see: http://www.johnkyrk.com/index.html Isoprenoids Isoprenoids • isoprenoids are widely distributed in the natural world – – particularly prevalent in plants and least common in insects; >30,000 known composed of integral numbers of C5 ‘isoprene’ units: • monoterpenes (C10); sesquiterpenes (C15); diterpenes (C20); sesterpenes (C25, rare); triterpenes (C30); carotenoids (C40) H O OH H O HO OH O OH thujone (C10) HO humulone (2x C5) lavandulol (C10) borneol (C10) (Z)--bisabolene (C15) H O O OH n O H ISOPRENOIDS natural rubber (~105x C5) H artemisinin (C15) O O OPP OPP -carotene (C40) H AcO H H HO cholesterol (C27 but C30-derived) BzHN OH O taxol (C20) OH HO BzO AcO H O HO H O O OH euonyminol (C15) H O HO HO O Ph H O isopentenyl pyrophosphate (IPP) dimethylallyl pyrophosphate (DMAPP) OH OH CO2H OH OH OH OH gibberellic acid (C20) (gibberellin A3) Biosynthesis of IPP & DMAPP - via Mevalonate • IPP & DMAPP are the key C5 precursors to all isoprenoids – the main pathway is via: acetyl CoA → acetoacetyl CoA → HMG CoA → mevalonate → IPP → DMAPP: Rational Anti-cholesterol Development - Statins • • HMG CoA → MVA is the rate determining step in the biosynthetic pathway to cholesterol ‘Statins’ inhibit HMG CoA reductase and are used clinically to treat hypercholesteraemia - a causative factor in heart disease, see: Wu et al. Tetrahedron 2015, 71, 8487 (DOI) – – e.g. mevinolin (=lovastatin®, Merck) from Aspergillus terreus is a competitive inhibitior of HMG-CoA reductase e.g. lipitor (Atorvastatin calcium, Pfizer) is also a competitive inhibitior of HMG-CoA reductase and the worlds biggest selling drug [first drug to reach $10 billion sales (2004: $10.8 bn] Linear C5n ‘head-to-tail’ Pyrophosphates • head-to-tail C5 oligomers are the key precursors to isoprenoids – – geranyl pyrophosphate (C10) is formed by SN1 alkylation of DMAPP by IPP → monoterpenes farnesyl (C15) & geranylgeranyl (C20) pyrophosphates are formed by further SN1 alkylations with IPP: Monoterpenes – -Terpinyl Cation Formation • geranyl pyrophosphate isomerises readily via an allylic cation to linalyl & neryl pyrophosphates – – (E) the leaving group abilty of pyrophosphate is enhanced by coordination to Mg2+ ions all three pyrophosphates are substrates for cyclases via an -terpinyl cation: OPP O O P O gerenyl pyrophosphate O O Mg P O O Mg OPP linalyl pyrophosphate OPP (Z) OPP cyclase = OPP neryl pyrophosphate initial chiral centre allylic cation intimate ion pair -terpinyl cation MONOTERPENES (C10) Monoterpenes – Fate of the -Terpinyl Cation • • The -terpinyl cation undergoes a rich variety of further chemistry to give a diverse array of monoterpenes Some important enzyme catalysed pathways are shown below – NB. intervention of Wagner-Meerwein 1,2-hydride- & 1,2-alkyl shifts -terpineol limonene hydrolysis trapping by PPO OH E1 elimination trapping with water a OPP c d c = He OPP b OPP H trapping by alkene at 'red' carbon (anti-Markovnikov) 1,2-alkyl shift H = H H O = = = -pinene H E1 elimination thujone E1 elimination E1 elimination d e O camphor camphene trapping by alkene at 'blue' carbon (Markovnikov) H2O 1,2-hydride shift OH borneol OPP bornyl pyrophosphate c d a -terpinyl cation [O] H H = b H - H = = -pinene Sesquiterpenes – Farnesyl Pyrophosphate (FPP) • ‘SN2’-like alkylation of geranyl PP by IPP gives farnesyl PP: pro-R hydrogen is lost OPP OPP HS HR (E) OPP geranyl PP • (E) OPP E,E-farnesyl PP (FPP) IPP just as geranyl PP readily isomerises to neryl & linaly PPs so farnesyl PP readily isomerises to equivalent compounds – allowing many modes of cyclisation & bicyclisation (E) (E) OPP O O P E,E-FPP (E) E,Z-FPP O (Z) O O Mg P O O cyclases OPP Mg 6-memb 10-memb 11-memb ring cyclised 'CATIONS' - further cyclisation - 1,2-hydride & alkyl shifts vast array of mono- & bicyclic SESQUITERPENES - trapping with H2O - elimination to alkenes OPP NB. control by: 1) enzyme to enforce conformation & sequestration of reactive intermediates 2) intrinsic stereoelectronics of participating orbitals nerolidyl PP allylic cation intimate ion pair Diterpenes – Geranylgeranyl PP → Taxol • Taxol is a potent anti-cancer agent used in the treatment of breast & ovarian cancers – – • comes from the bark of the pacific yew (Taxus brevifolia) binds to tubulin and intereferes with the assembly of microtubules biosynthesis is from geranylgeranyl PP: cembrene a cyclisation b AcO 14-exo OPP geranylgeranyl PP H a H O BzNH O taxol H OPP HO sesquiterpene (isoprenoid) OH Ph b O HO BzO AcO O -amino acid (shikimate) – – for details see: http://www.chem.qmul.ac.uk/iubmb/enzyme/reaction/terp/taxadiene.html home page is: http://www.chem.qmul.ac.uk/iubmb/enzyme/ • • recommendations of the Nomenclature Committee of the International Union of Biochemistry and Molecular Biology on the Nomenclature and Classification of Enzyme-Catalysed Reactions based at Department of Chemistry, Queen Mary University of London Triterpenes – FPP → Squalene • triterpenes (C30) arise from the ‘head to head’ coupling of two fanesyl PP units to give squalene catalysed by squalene synthase: OPP FPP (acceptor) OPP FPP (donor) – – squalene was first identified as a steroid precursor from shark liver oil the dimerisation proceeds via an unusual mechanism involving electrophilic cyclopropane formation rearrangement to a tertiary cyclopropylmethyl cation and reductive cyclopropane ring-opening by NADPH (NB. exact mechanism disputed) EnzB: H H OPP OPP blocked by squalestatins squalene synthase H presqualene PP OPP – Zaragozic acids (squalestatins) mimic a rearrangement intermediate and inhibit squalene synthase. They constitute interesting leads for development of new treatments for hypercholesteraemia & heart disease (cf. statins) H H OPP NADPH NADP O OH O HO2C zaragozic acid A HO2C (squalestatin S1) O O OH CO2H + PPi H H OAc squalene Oxidosqualene-Lanosterol Cyclase – Mechanism • oxidosqualene-lanosterol cyclase catalyses the formation of lanosterol from 2,3-oxidosqualene: – – – this cascade establishes the characteristic ring system of ALL steroids ring-expansion sequence to establish the C ring the process is NOT concerted, discrete cationic intermediates are involved & stereoelectronics dictate the regio- & stereoselectivity although the enzyme undoubtedly lays a role in pre-organising the ~chair-boatchair conformation – “The enzyme’s role is most likely to shield intermediate carbocations… thereby allowing the hydride and methyl group migrations to proceed down a thermodynamically favorable and kinetically facile cascade” • Wendt et al. Angew. Chem. Int. Ed. 2000, 39, 2812 (DOI) & Wendt ibid 2005, 44, 3966 (DOI) Lanosterol → Cholesterol – Oxidative Demethylation • Several steps are required for conversion of lanosterol to cholesterol: 24 24 hydrogenation H 8 1 4 HO 24 2) 14 DEMETHYLATION H 14 HO 3) 4 & 4DEMETHYLATION 5 H to rearrangement 8 H = H 5 H flat, rigid structure HO lanosterol cholesterol 2) 14 DEMETHYLATION hydrogenation NADPH H 2x O2 P450 H O2 P450 HCO2H H H NADPH H 24 14 2x H2O NADP O H O HO O H :BEnz NADP FeIII 3) 4 & 4DEMETHYLATION 4x O2 HO 4x O2 P450 4 O H 4x H2O O H O O CO2 5 4x H2O isomerase H 5 H H O H O O CO2 NADH NAD 8 NADPH O H H to rearrangement 8 O H P450 H H NADH [-> vitamin D] NAD HO H NADP H Cholesterol → Human Sex Hormones • cholesterol is the precursor to the human sex hormones – progesterone, testosterone & estrone – – the pathway is characterised by extensive oxidative processing by P450 enzymes estrone is produced from androstendione by oxidative demethylation with concomitant aromatisation: OH OH H 2x O2 P450 H H H O O2 P450 H H H HO 2x H2O cholesterol H O NAD H H H O H HO H H H NADH HO H O progesterone [O] O FeIII EnzB: H H H X O O O OH O2 P450 O H H H HO estrone (œstrone) HCO2H O 2x O2 H H P450 H H O DEMETHYLATIVE aromatisation by 'aromatase' enzyme H O 2x H2O androstendione (X = O) testosterone (X = H, OH) Steroid Ring Cleavage - Vitamin D & Azadirachtin • • vitamin D2 is biosynthesised by the photolytic cleavage of 7-dehydrocholesterol by UV light: – a classic example of photo-allowed, conrotatory electrocyclic ring-opening: – D vitamins are involved in calcium absorption; defficiency leads to rickets (brittle/deformed bones) Azadirachtin is a potent insect anti-feedant from the Indian neem tree: – exact biogenesis unknown but certainly via steroid modification: O O MeO2C O O OAc H O OH C H 11 12 OH O 14 O 7 HO H OH AcO H AcO tirucallol (cf. lanosterol) H OH H azadirachtanin A (a limanoid = tetra-nor-triterpenoid) oxidative cleavage of C ring 8 AcO MeO2C H OH O azadirachtin O O OH highly hindered C-C bond for synthesis! Summary of Presentation • Metabolism & Biosynthesis – • Shikimate Metabolites – – • acetylCoA & the citric acid cycle → -amino acids → alkaloids Opioids – powerful pain killers Fatty Acids and Polyketides – – • photosynthesis & glycolysis → shikimate formation → shikimate metabolites Glyphosate – a non-selective herbicide Alkaloids – – • some definitions, 1° & 2° metabolites acetylCoA → malonylCoA → fatty acids, prostaglandins, polyketides, macrolide antibiotics NSAIDs – anti-inflammatory’s Isoprenoids/terpenes – – acetylCoA → mevalonate → isoprenoids, terpenoids, steroids, carotenoids Statins – cholesterol-lowering agents