* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Utilization and Transport of Mannitol in Olea
Extracellular matrix wikipedia , lookup
Cell growth wikipedia , lookup
Cytokinesis wikipedia , lookup
Endomembrane system wikipedia , lookup
Tissue engineering wikipedia , lookup
Cellular differentiation wikipedia , lookup
Cell culture wikipedia , lookup
Cell encapsulation wikipedia , lookup
Magnesium transporter wikipedia , lookup
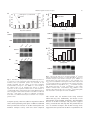
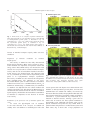
Plant Cell Physiol. 48(1): 42–53 (2007) doi:10.1093/pcp/pcl035, available online at www.pcp.oxfordjournals.org ß The Author 2006. Published by Oxford University Press on behalf of Japanese Society of Plant Physiologists. All rights reserved. For permissions, please email: [email protected] Utilization and Transport of Mannitol in Olea europaea and Implications for Salt Stress Tolerance Carlos Conde 1, Paulo Silva 1, Alice Agasse 1, Rémi Lemoine 2, Serge Delrot 2, Rui Tavares 1 and Hernâni Gerós 1, * 1 Departamento de Biologia, Universidade do Minho, Campus de Gualtar, 4710-057 Braga, Portugal UMR CNRS 6161, Transport des Assimilats, Laboratoire de Physiologie, Biochimie et Biologie Mole´culaires Ve´ge´tales, Bâtiment Botanique, UFR Sciences, 40 Avenue du Recteur Pineau, 86022 Poitiers Ce´dex, France 2 Introduction Mannitol is one of the primary photosynthetic products and the major phloem-translocated carbohydrate in Olea europaea L., an important crop in the Mediterranean basin. Uptake of mannitol in heterotrophic cell suspensions of O. europaea was shown to be mediated by a 1 : 1 polyol : Hþ symport system with a Km of 1.3 mM mannitol and a Vmax of 1.3 nmol min1 mg1 DW. Dulcitol, sorbitol and xylitol competed for mannitol uptake, whereas glucose and sucrose did not. Reverse transcription–PCR (RT–PCR) performed on mRNA extracted from cultured cells exhibiting high mannitol transport activity allowed the cloning of a partial O. europaea mannitol carrier OeMaT1. The Vmax of mannitol uptake and the amount of OeMaT1 transcripts increased along with polyol depletion from the medium, suggesting that the mannitol transport system may be regulated by its own substrate. Addition of 100–500 mM NaCl to cultured cells enhanced the capacity of the polyol : Hþ symport system and the amount of OeMaT1 transcripts, whereas it strongly repressed mannitol dehydrogenase activity. Measurements of cell viability showed that mannitol-grown cells remained viable 24 h after a 250 and 500 mM NaCl pulse, whereas extensive loss of cell viability was observed in sucrose-grown cells. OeMaT1 transcripts increased throughout maturation of olive fruits, suggesting that an OeMaT is involved in the accumulation of mannitol during ripening of olive. Thus, mannitol transport and compartmentation by OeMaT are important to allocate this source of carbon and energy, as well as for salt tolerance and olive ripening. Polyols (or sugar alcohols), the reduced form of aldoses and ketoses, can be either cyclic (cyclitols) or linear (alditols), and are present in all living forms. In some plant species, polyols are direct products of photosynthesis in mature leaves, together with sucrose. Mannitol is the most widely distributed sugar alcohol in nature and has been reported in 4100 species of vascular plants of several families, including the Rubiaceae (coffee), Oleaceae (olive, privet) and Apiaceae (celery, carrot, parsley). Mannitol is synthesized in mature leaves from mannose6-phosphate, through the combined action of a NADPH-dependent mannose-6-phosphate reductase and a mannitol-6-phosphate phosphatase. It is transported to sink tissues where it can be either stored or oxidized to mannose by an NAD-dependent mannitol dehydrogenase (MTD) (reviewed by Stoop et al. 1996, Noiraud et al. 2001b). In contrast to sucrose and monosaccharide transporters, little is known regarding the identity and regulation of polyol transporters either in sink or in source tissues in higher plants. The first cDNA encoding a mannitol transporter of a higher plant was identified and characterized in celery phloem (Noiraud et al. 2001a). This cDNA (AgMaT1, Apium graveolens mannitol transporter 1) was used to establish a heterologous expression system in yeast cells. In the past 5 years, polyol transporters from Prunus cerasus (Gao et al. 2003), Plantago major (Ramsperger-Gleixner et al. 2004), Malus domestica (Watari et al. 2004) and Arabidopsis thaliana (Klepek et al. 2005, Schneider et al. 2006) have been characterized. Mannitol production may confer several potential advantages including more efficient carbon use (Stoop et al. 1996), resistance against oxidative stress (Smirnoff and Cumbers 1989, Williamson et al. 1995, Jennings et al. 1998) and salt tolerance. Thus, the mannitol concentration in celery grown in hydroponic nutrient solution progressively Keywords: Mannitol — Olea europaea — Polyol transport — Salt stress. Abbreviations: CCCP, carbonyl cyanide m-chlorophenylhydrazone; FDA, fluorescein diacetate; MTD, mannitol dehydrogenase; mltD, mannitol-1-phosphate dehydrogenase; PI, propidium iodide; TPPþ, tetraphenylphosphonium; RT–PCR, reverse transcription–PCR. *Corresponding author: E-mail, [email protected]; Fax, þ 351-253678980. 42 Mannitol uptake in Olea europaea increases as the total salinity of the growth solution increases (Stoop and Pharr 1994). Increased mannitol accumulation in leaves was also observed in plants irrigated with 0.3 M NaCl, as a consequence of a massive shift in partitioning of fixed carbon into mannitol instead of sucrose (Everard et al. 1994). The strong water stress tolerance of Fraxinus excelsior is in part related to an accumulation of malate and mannitol (Guicherd et al. 1997), and, in plants subjected to drought stress, the mannitol content of the leaf xylem sap increases (Patonnier et al. 1999). Additional evidence for a role for mannitol in salinity tolerance was obtained when Nicotiana tabacum, Populus tomentosa and other plants were genetically engineered to synthesize mannitol through introduction of an Escherichia coli mannitol-1-phosphate dehydrogenase (mltD), which catalyzes the biosynthesis of mannitol from fructose, resulting in more salt-tolerant plants (Tarczynsky et al. 1993, Hu et al. 2005). In Arabidopsis, mltD gene transfer and expression enhanced seed germination under salinity conditions (Thomas et al. 1995). Olea europaea L. is an evergreen moderately salttolerant tree (Therios and Misopolinos 1988, Rugini and Fedeli 1990) traditionally cultivated in the Mediterranean basin. Olives and olive oil play an increasingly important nutritional role and are an essential part of what is now widely known as the ‘Mediterranean diet’. Sugars are the main soluble components in olive tissues and play important roles, providing energy and acting as precursors for olive oil biosynthesis. Glucose, fructose and galactose are the main sugars found in olive pulp, but appreciable quantities of mannitol are also present (Marsilio et al. 2001). Water deficit, freezing, salinity and air pollution are a few of the stress factors restricting plant growth, so that olive productivity at the end of the growing season expresses only a fraction of the plant’s genetic potential (Vitagliano and Sebastiani 2002). In this context, the elucidation of the role of mannitol as carbon and energy source for plant growth and as a protecting solute 43 against salinity and drought is important for the improvement of yield potential of the plant. The present work characterizes a mannitol transporter expressed in cultured cells and intact fruits of O. europaea. For the first time in plants it is shown that mannitol transport is regulated by means of salt-mediated changes in the transcription of mannitol carrier(s). Altogether, the results showed that transmembrane transport of mannitol is a critical step in terms of osmotic adjustments and productivity in O. europaea. Results Growth in batch cultures with mannitol Suspension cell cultures mimic heterotrophic plant tissues, where carbohydrates are imported from photosynthetically active source tissues, thus making them a suitable model system to study sugar transport into sink cells. The capacity of O. europaea cell suspensions to use sucrose, lactose, glucose, galactose, fructose, mannitol and glycerol as sole carbon and energy sources was studied previously (Oliveira et al. 2002). Among these substrates, only lactose and glycerol were unable to promote cell growth. Growth of O. europaea cell suspensions with 1% (w/v) mannitol and its consumption are depicted in Fig. 1A. To ascertain whether mannitol transport constitutes the rate-limiting step of the growth of O. europaea cells on mannitol, the growth parameters were compared with the maximal capacity of mannitol uptake. The following values for the maximum specific growth rate (mmax) and yield coefficient (Y ) were estimated: 0.2 d1 and 0.6 g biomass g1 mannitol, respectively. From the ratio mmax/Y, a value for the specific mannitol transfer rate (q) of 1.28 nmol min1 mg1 DW was estimated, similar to the corresponding Vmax of mannitol transport (Table 1), suggesting that mannitol uptake is an important metabolic step for the control of cell growth. Growth of O. europaea cells in a medium containing a mixture of 0.5% (w/v) glucose and 0.5% (w/v) mannitol is depicted in Fig. 1B. The rate of glucose Fig. 1 Representative growth curves of O. europaea cells in MS medium with 1% (w/v) mannitol (A) and with 0.5% (w/v) glucose and 0.5% (w/v) mannitol (B). 44 Mannitol uptake in Olea europaea Table 1 Specific rate of mannitol transfer (q), D-[14C]mannitol uptake and MTD activity in suspension-cultured cells of O. europaea cultivated with 1% (w/v) mannitol D-[ q Mid-exponential growth phase ([mannitol]medium 0.5%) Late exponential growth phase ([mannitol]medium 550.1%) Salt stressedd 1 14 MTD activityc C]mannitol uptake 1.40 0.07a (0.25 0.05)b 0.22 0.02 1.28 1.29 0.04b 0.47 0.11 – 1.5 0.08b 0.09 0.01 1 Units are in nmol mannitol min mg DW. Values are the means SD of three independent experiments. a [mannitol]ext ¼ 20 mM (Fig. 2B). b Vmax of the mannitol:Hþ symport system. c Vmax of MTD. d 24 h after 500 mM NaCl addition, as indicated in Fig. 6. depletion from the medium was higher than that of mannitol; after glucose exhaustion (day 8), mannitol sustained cell growth up to day 12. 1.5 A 1.0 1.5 v 1.0 v (nmol min−1mg−1D.W.) 0.5 0.5 0.0 0.0 0.25 0.50 0.75 1.00 v/[S] 0.0 1.5 B 1.0 1.5 v Mannitol transport Transport experiments were conducted in O. europaea cell suspensions harvested at the end of the exponential growth phase, after 10–15 d in culture, when the mannitol concentration had fallen to about 0.05% (w/v). Initial uptake rates of 0.2–20 mM D-[14C]mannitol followed Michaelis–Menten kinetics (Fig. 2A), suggesting carriermediated transport. By the application of a computerassisted non-linear regression analysis (GraphPad Prism, version 4.0) to the data, the following kinetic parameters were obtained: Km, 1.3 0.15 mM mannitol and Vmax, 1.29 0.04 nmol mannitol min1 mg1 DW. Cells of O. europaea cultivated with 1% mannitol, collected at the mid-exponential growth phase when the mannitol concentration in the medium is approximately 0.5% (w/v) (see Fig. 1A), also displayed the capacity to transport mannitol; however, the Eadie–Hofstee plot of 14 D-[ C]mannitol initial uptake rates was biphasic (Fig. 2B). The computer-assisted non-linear regression analysis of the data agreed with the presence of two distinct transport modes: saturating transport associated with first order kinetics. The following values were estimated for the maximal capacity of saturating transport and for the diffusion-like component: Vmax, 0.25 0.05 nmol mannitol min1 mg1 DW; kd, 0.06 0.01 ml min1 mg1 DW. To study the specificity of the transport system, the initial uptake rates of 0.1–2 mM D-[14C]mannitol were estimated in the presence of the following unlabeled putative competitors: dulcitol, sorbitol, xylitol, myoinositol, glucose, fructose, mannose and sucrose. The acyclic polyols dulcitol, sorbitol and xylitol behaved as competitive inhibitors (Fig. 3A), suggesting that they share the same carrier; glucose and the remaining substrates (not shown) had no effect on the transport of mannitol, 0.5 1.0 0.5 0.0 0.0 0.25 0.50 v/[S] 0.75 0.0 0 5 10 [D-Mannitol] (mM) 15 20 Fig. 2 Mannitol transport by suspension-cultured cells of O. europaea cultivated with 1% mannitol as in Fig. 1A. Initial uptake rates of D-[14C]mannitol, at pH 4.5, by cells collected at the end of the exponential growth phase ([mannitol]medium 550.1%, w/v) (A) and at mid-exponential growth phase ([mannitol]medium 0.5%, w/v) (B). Inserts: Eadie–Hofstee plots of the initial D-[14C]mannitol uptake rates. thus appearing not to be recognized by the permease. Although the identified transport system seems to be specific for polyols, O. europaea cell suspensions cultivated in similar conditions are able to transport glucose according Mannitol uptake in Olea europaea v (nmol min−1 mg−1 D.W.) 0.75 0.9 A 0.50 0.6 0.25 0.3 0.00 0.0 0.25 0.50 45 0.0 0.0 0.75 B 2.5 5.0 7.5 10.0 v / [S] 14 Fig. 3 Eadie–Hofstee plots of the initial uptake rates, at pH 4.5, of D-[ C]mannitol (A) and D-[14C]glucose (B) by suspension-cultured cells of O. europaea. Transport was measured in the absence of other sugars or polyols (filled boxes) and in the presence of unlabeled 5 mM sorbitol (filled inverted triangles), 5 mM dulcitol (filled triangles), 5 mM xylitol (filled diamonds), 20 mM mannitol (open triangles) and 20 mM glucose (open squares). Cells were cultivated with 1% (w/v) mannitol as in Fig. 1A and collected at the end of the exponential growth phase as described in Fig. 2A. to a carrier-mediated mechanism with Km ¼ 67 30 mM glucose and Vmax ¼ 1.45 0.26 nmol glucose min1 mg1 DW (Oliveira et al. 2002). Here, inhibition experiments of 14 D-[ C]glucose uptake by different polyols were performed to check that polyol uptake does not occur via the monosaccharide transport system. Mannitol at 20 mM did not inhibit 0.02–0.5 mM D-[14C]glucose uptake (Fig. 3B). Similar results were obtained with sorbitol and dulcitol (data not shown). Taken together, the results suggest that, in O. europaea, monosaccharides and polyols are transported via two distinct transport systems with different Kms. This could account for the results depicted in Fig. 1B, where glucose is the first substrate to be consumed when growth occurred in a medium with glucose and mannitol. To study the energetics of mannitol transport in O. europaea cultured cells, D-[14C]mannitol uptake was measured at different external pH values. Vmax decreased abruptly from pH 4.5 to 5.5, with little activity remaining above pH 5.5, consistent with the involvement of a proton-dependent transport system (Fig. 4A). Additionally, the uptake of 0.2–2.0 mM [14C]mannitol was strongly inhibited by 50 mM of the protonophore m-chlorophenylhydrazone (CCCP) (Fig. 4B). The occurrence of transient alkalinization of extracellular media upon addition of mannitol to cell suspensions (Fig. 4C) provided clear evidence for the involvement of a mannitol–proton symport system. The initial velocities of proton uptake were estimated from the slope of the initial part of the pH trace (the alkalinization curve) after 5 mM mannitol (saturating concentrations) had been added. A value of 1.1 nmol Hþ min1 mg1 DW can be obtained from the data of Fig. 4C, similar to the maximal capacity of the mannitol : Hþ symport system measured with D-[14C]mannitol, suggesting a 1 mannitol : 1 proton stoichiometry. Since such a mechanism would be associated with a net influx of positive charges into the cells, the effect of the dissipation of transmembrane electric potential on mannitol transport was studied. Fig. 4B shows the effect of the lipophilic cation tetraphenylphosphonium (TPPþ) on the initial uptake rates of 0.2–2 mM D-[14C]mannitol. TPPþ inhibited mannitol uptake, indicating that membrane potential makes a significant contribution to the driving force for substrate transport by the polyol carrier. Polyol : Hþ symport activity and OeMaT expression As the Vmax of the O. europaea polyol : Hþ symport system increased along with mannitol depletion from the medium throughout the exponential growth (see Fig. 2), mannitol levels appear to have a regulatory effect. To study the induction of transport activity in response to mannitol concentration, the accurate dependence of permease activity on polyol levels in the medium was evaluated. Cells were grown in a medium with 1% (w/v) mannitol, and the uptake of D-[14C]mannitol was measured in cell aliquots harvested from the culture at the times indicated in Fig. 5. When the external levels of mannitol fell below 0.05% (w/v) (day 13), the activity of the polyol transport system increased abruptly from basal levels. Maximal activity was observed when mannitol was completely exhausted from the culture medium (day 15). The addition of 0.05 mg ml1 cycloheximide to the culture medium prevented the increase of mannitol transport activity, suggesting the involvement of de novo protein synthesis. To identify the cDNA sequence encoding the O. europaea mannitol : Hþ symport system, degenerated primers corresponding to conserved regions of polyol transporters were used, and reverse transcription–PCR 46 Mannitol uptake in Olea europaea Vmax (nmol min−1 mg−1 D.W.) 0.75 A 1.2 1.0 B 0.50 0.8 0.25 0.6 0.4 4.5 5.0 5.5 6.0 6.5 0.00 0.00 7.0 0.25 pH 0.50 0.75 v/(S) C Alkalinization (10 nmol H+) 10 s 2 mM mannitol Fig. 4 Energetics of the O. europaea polyol transport system. (A) pH dependence of mannitol transport. (B) Eadie–Hofstee plots of the initial uptake rates of D-[14C]mannitol in the absence (filled squares) or presence of 50 mM CCCP (open circles) and 10 mM TPPþ (filled triangles). (C) Proton movements, at pH 4.5, associated with the addition of 2 mM mannitol to cell suspensions. Cells were cultivated with 1% (w/v) mannitol as in Fig. 1A and collected at the end of the exponential growth phase as described in Fig. 2A. (RT–PCR) was performed on mRNA extracted from O. europaea suspension-cultured cells exhibiting high mannitol transport activity. This allowed the cloning of a 501 bp cDNA OeMaT1 (accession No. DQ059507) with extensive homology to the celery mannitol transporter AgMaT2. Low-stringency Southern blots of O. europaea genomic DNA digested with four different restriction enzymes and hybridized with OeMaT1 cDNA, identified one (HindIII and XhoI) and three (XbaI and EcoRI) bands (Fig. 5D). This suggests the presence of a multigene family of polyol transporters in olive tree, which is consistent with evidence available for other plant species such as P. cerasus (Gao et al. 2003), P. major (RamspergerGleixner et al. 2004), M. domestica (Watari et al. 2004) and A. thaliana (Klepek et al. 2005). An OeMaT1 partial cDNA sequence was used as a probe for Northern analysis (Fig. 5B). Due to the impossibility of obtaining the full-length cDNA, we were not able to design a specific probe. We must therefore consider the possibility of a high level of sequence identity with the other potential mannitol transporters present in the O. europaea genome with the probable occurrence of cross-hybridization. For the rest of the work, we will refer to OeMaT to indicate that the signals observed in Northern experiments were either due to OeMaT1 or to other unidentified mannitol transporters present in O. europaea. The parallel between OeMaT transcripts and mannitol transport activity shown in Fig. 5B suggests that carrier expression is mainly controlled at the transcriptional level, although other forms of post-transcriptional regulation cannot be ruled out. These results show for the first time a tight regulation of a mannitol : Hþ symport expression by external levels of its own substrate. As referred to in the Introduction, substantial amounts of mannitol are present in the pulp of olive fruit, reaching maximum values in ripened olives of 8 mg g1 DW (Marsilio et al. 2001). To study the involvement of the polyol : Hþ symport system in mannitol unloading during olive fruit maturation, RNAs were isolated from olive fruits at the green, cherry and black stages, and the expression of OeMaT was studied. Although detectable throughout fruit development, OeMaT transcript levels strongly increased during the late stage of the ripening process (black stage) at the onset of mannitol accumulation (Fig. 5C). Effect of salt stress on polyol : Hþ symport activity and OeMaT expression As referred to in the Introduction, mannitol can act as a compatible solute besides its role as a carbon and energy source. To study the influence of salt stress on mannitol ( ) Mannitol (%, w/v) 1.2 0.05 mg mL−1 cyclohexamide Control 0.5 0.4 0.9 0.3 0.6 0.2 0.3 0.1 0.0 Vmax (nmol min−1 mg−1 D.W.) A Vmax (nmol min−1 mg−1 D.W.) Mannitol uptake in Olea europaea A 1.5 10.0 13.0 14.0 14.5 Days after subculture 0 mM NaCl 500 mM NaCl 1.0 0.5 0.0 0.0 7.0 47 0 15.0 6 12 24 Time (h) B OeMaT 1 OeMaT1 0 mM NaCl OeMaT1 500 mM NaCl rRNA Vmax (nmol min−1 mg−1 D.W.) Bl ac k rry he G re C C en rRNA B OeMaT 1 III Ec oR I Xh oI (bp) H in d D Xb al rRNA 1.5 1.0 0.5 0.0 0.0 11490 100.0 250.0 300.0 [NaCl] (mM) 500.0 OeMaT1 2560 rRNA 1159 Fig. 5 Mannitol : Hþ symport activity and OeMaT expression in O. europaea. (A) Cell suspensions were cultivated with 1% (w/v) mannitol, and D-[14C]mannitol uptake was measured in cell aliquots harvested from the culture at the times indicated. Cycloheximide (0.05 mg ml1) was added to a 25 ml aliquot of the culture at day 14. (B) Northern blot analysis of OeMaT expression in the suspension-cultured cells in the absence of cycloheximide and (C) in developing olive fruit at different stages of olive maturation. Total soluble mannitol concentration in the fruit pulp (mg g1 DW): green, 5.5; cherry, 5.5; and black, 8.0 (Marsilio et al. 2001). (D) Southern blot analysis of OeMaT1 in O. europaea genomic DNA. transport capacity, NaCl was added to suspension-cultured cells at mid-exponential growth phase. Addition of 500 mM NaCl promoted the increase of OeMaT transcription and mannitol transport activity with time when compared Fig. 6 Effect of salt stress on O. europaea mannitol : Hþ symport activity and OeMaT expression. (A) Time dependence of Vmax of mannitol transport and OeMaT transcripts upon addition of 500 mM NaCl. Cells were grown with 1% (w/v) mannitol as in Fig. 5, NaCl was added to 25 ml aliquots of the culture at day 8 and 14 D-[ C]mannitol uptake was measured in cell aliquots at the time periods indicated. (B) Dependence of Vmax of mannitol transport and OeMaT transcripts on NaCl concentration in the culture medium. Different NaCl concentrations were added to 25 ml aliquots of the culture at day 8 as in A, and D-[14C]mannitol uptake was measured in cell aliquots 24 h after salt addition. with control cells, the maximal levels being achieved within 24 h (Fig. 6A). Additionally, the increase of salt concentration in the medium promoted a dose-dependent increase of OeMaT transcripts and polyol : Hþ symport activity, measured after 24 h (Fig. 6B). The parallel between Vmax of mannitol transport and OeMaT message levels suggests that the expression of OeMaT is responsible for the 48 Mannitol uptake in Olea europaea A - Mannitol-grown cells 0 mM NaCl 500 mM NaCl 250 mM NaCl 500 mM NaCl Visible 0.4 250 mM NaCl 0.005 0.010 0.015 v/[S] Fig. 7 MTD activity of O. europaea suspension-cultured cells. Cells were grown with 1% (w/v) mannitol as in Fig. 1 and collected at mid-exponential growth phase, as described in Fig. 2B ([mannitol]medium 0.5%, w/v) (filled circles) and at the end of the exponential growth phase, as indicated in Fig. 2A ([mannitol]medium 550.1%, w/v) (filled squares). MTD activity of salt-stressed cells measured 24 h after 500 mM NaCl addition, as described in Fig. 6 (filled triangles). increase of mannitol transport capacity under salt stress conditions. Regulation of mannitol catabolism by mannitol dehydrogenase The activity of MTD has been fully characterized during the last decade by Pharr and co-workers in celery, where mannitol also represents an important carbon and energy source. Here, MTD activity was measured in crude extracts of O. europaea cells to correlate mannitol transport activity with the rate of intracellular mannitol conversion. Crude extracts were obtained from cells cultivated with 1% (w/v) mannitol up to mid- and late exponential growth phase as in D-[14C]mannitol transport experiments. The activity of MTD was also measured in homogenates from mannitol-grown cells subjected to salt stress. The Eadie–Hofstee plots of initial velocities of mannitol oxidation were linear over 5–150 mM mannitol, and a Km of 40 mM was obtained (Fig. 7). MTD activity was enhanced as mannitol was depleted from the culture medium and strongly repressed by salt. The decrease of MTD activity in salt-stressed cells associated with the increase of mannitol uptake capacity should allow the intracellular accumulation of mannitol to compensate for the decrease of external water activity. Evaluation of the protective role of mannitol against salt stress To assess the physiological role of mannitol in salt stress tolerance in O. europaea, we studied cell viability after salt addition to mannitol-grown cells and B - Sucrose-grown cells 0 mM NaCl Visible 0.0 0.000 Epifluorescence 0.2 Epifluorescence v (µmol h−1 mg−1 protein) 0.6 Fig. 8 Cell viability assays in O. europaea suspension-cultured cells cultivated with mannitol (A) and sucrose (B) 24 h after the addition of 250 and 500 mM NaCl. Fluorescence was measured after incubation with fluorescein diacetate (FDA, green fluorescence) and propidium iodide (PI, red fluorescence). sucrose-grown cells. Cell aliquots were collected from each medium at mid-exponential growth phase, and 250 and 500 mM NaCl were added. Cell viability was assessed by fluorescein diacetate (FDA) and propidium iodide (PI). The intact plasma membrane is permeable to FDA, and FDA is converted to a green fluorescent dye, fluorescein, by a function of internal esterases, showing a green color in viable cells. In contrast, the intact plasma membrane is impermeable to PI. Damaged cells having pores on the plasma membrane incorporate the dye, which binds to genomic DNA and generates a red fluorescence (Jones and Senft 1985). Fig. 8 shows that a large population of Mannitol uptake in Olea europaea mannitol-grown cells remained viable 24 h after a 500 mM NaCl pulse. Sucrose-grown cells were not able to display green fluorescence after the same treatment, and showed extensive loss of cell viability as evaluated by the PI red fluorescence. A similar result was obtained with glucosegrown cells (not shown). Taken together, these results suggest that mannitol plays essential roles in O. europaea, both providing a carbon and energy source for sink tissues and acting as an osmoprotectant in response to high salinity. Discussion Mannitol is the most abundant carbohydrate in olive tree leaves, accounting for 82–92% of the total soluble carbon (Drossopoulos and Niavis 1988), and is an important sugar in the olive fruit pulp (Marsilio et al. 2001). However, very little information is available regarding the utilization and transport of mannitol in this polyol-synthesizing plant. In the present work, mannitol transport mechanisms operating in O. europaea heterotrophic cells were investigated in detail. In addition, the regulation of the expression of the polyol transport system and its relevance in osmotic adjustments was also studied. The data obtained are particularly relevant because the olive tree is normally cultivated in areas in which water is the main limiting factor in agricultural production (Tattini et al. 1994). Although it is recognized that suspensioncultured cells may not be close to normal physiological conditions, they provide a convenient experimental system that has already yielded a lot of useful information on sugar transport mechanisms and regulation (Roitsch and Tanner 1994, Ehness et al. 1997, Oliveira et al. 2002, Cakir et al. 2003, Azevedo et al. 2006, Conde et al. 2006). Mannitol-grown cells of O. europaea exhibit a specific growth rate (mmax, 0.2 d1) higher than cells cultivated with glucose (mmax, 0.07 and 0.11 d1 in media with 0.5 and 3% glucose, respectively; Oliveira et al. 2002) or 2% sucrose (mmax, 0.08 d1), possibly because mannitol catabolism produces a higher number of ATPs than the catabolism of an equal amount of glucose or sucrose. From the linear part of the growth curve, a value for the yield coefficient (Y ) of 0.6 g biomass g1 mannitol was estimated, suggesting that most of the carbohydrate is respired and used as a carbon and energy source for exponential growth. Therefore, little or no sugar is channeled to internal stores, which is confirmed by the arrest of cell growth that was associated with the decline of sugar content. The fact that mannitol behaved as an efficient carbon and energy source in heterotrophic cells of O. europaea is consistent with the role of this compound as a major photoassimilate in this species. 49 The saturable transport observed in O. europaea cells involves a polyol : Hþ symport system with a stoichiometry of 1 mannitol : 1 Hþ as indicated by the following observations: (i) mannitol addition to weakly buffered cell suspensions is associated with a transient alkalinization of the extracellular medium; (ii) the Vmax of proton uptake is similar to the Vmax of carrier-mediated D-mannitol uptake and depended on extracellular pH; (iii) dissipation of the proton-motive force by CCCP significantly inhibited the initial velocities of D-mannitol uptake; and (iv) mannitol transport was sensitive to TPPþ, suggesting that the is an important component of the proton-motive force involved in mannitol accumulation. Proton dependence and substrate affinity (Km, 1.3 mM mannitol) are in good agreement with the data obtained for the celery mannitol transporter. In this polyol-producing plant, different membrane transport steps have been studied, from phloem loading to phloem unloading and storage in parenchyma cells. The cloned AgMaT1 gave yeast cells the ability to grow on mannitol, and a Km value for mannitol uptake of 0.34 mM was obtained (Noiraud et al. 2001a), that correlates well with the value (Km, 0.64 mM) determined in plasma membrane vesicles from phloem strands of celery (Salmon et al. 1995). The involvement of a co-transport with protons was proposed because the uptake of mannitol was almost abolished by CCCP and was maximal at acidic pH. Also, in storage parenchyma discs of celery leaves and in plasma membrane vesicles from parenchyma cells, Km values of 1 mM were obtained (Keller 1991, Salmon et al. 1995). In contrast, mannitol transport in vacuoles of celery parenchyma cells seems to be mediated by facilitated diffusion, because it was neither stimulated by energization with ATP and pyrophosphate, nor impaired by the dissipation of the proton-motive force (Greutert et al. 1998). The selectivity of the O. europaea polyol : Hþ symport system is rather poor for mannitol because the transport system was able to accept, besides mannitol, dulcitol, sorbitol and xylitol, as these acyclic polyols behaved as competitive inhibitors. In addition, monosaccharide uptake and polyol uptake are mediated by two distinct transport systems since glucose did not inhibit D-[14C]mannitol uptake. Likewise, mannitol did not affect D-[14C]glucose uptake mediated by a monosaccharide : Hþ symport system previously identified in mannitol-grown cells (Oliveira et al. 2002). Accordingly, glucose is not transported by Saccharomyces cerevisiae expressing the celery mannitol transporter AgMaT1 (Noiraud et al. 2001a). This is in contrast to the polyol transporter from Arabidopsis AtPST5 which can also transport glucose (Klepek et al. 2005). Kinetic parameters may explain why D-glucose is consumed before mannitol when the two substrates are present in the extracellular medium: the capacity of both 50 Mannitol uptake in Olea europaea activity increased, suggesting that mannitol regulates carrier expression at the transcriptional level, although other levels of regulation cannot be ruled out. Salt stress can be regarded as a situation in which plants have to cope with both decreased water availability and ion toxicity (Lewitt 1980). Despite numerous studies indicating that, following polyol synthesis in mature leaves, there is an increase in polyol content in sink organs in response to drought or salt stress (see Introduction), and that salt alters enzyme activities related to polyol metabolism (Stoop and Pharr 1994, Williamson et al. 1995), little is known about the regulation of the expression of polyol transporters under such conditions. In Mesembryanthemum crystallinum, where the genes MITR1 and MITR2 behave as myo-inositol : Naþ symporters, myo-inositol could be a signal during the adaptation to salt stress (Nelson et al. 1999, Chauhan et al. 2000). In celery plants subjected to severe salt stress, the expression of AgMaT1 has not been studied yet, but it appears that the expression of the sucrose carrier AgSUT1 decreased in all organs (Noiraud et al. 2000), suggesting that transport of mannitol is favored. The present work provides clear evidence that O. europaea suspension-cultured cells exposed to high salinity display an increase of OeMaT expression and mannitol transport activity, together with a drastic decrease of mannitol oxidation by MTD. This should allow the intracellular accumulation of mannitol in order to compensate the decrease of external water activity, providing a tolerance mechanism to salinity in O. europaea (Fig. 9). Similarly, celery plants exposed to high salinity showed a specific down-regulation of MTD activity in sink tissues, resulting in decreased mannitol use and an accompanying increase in mannitol accumulation (Stoop and Pharr 1994, Pharr et al. 1995), and in cell suspensions, Mtd transcripts decreased in parallel with MTD activity upon addition of NaCl (Williamson et al. 1995). The present work demonstrates the importance of mannitol as a carbon and energy source in O. europaea, as well as its role in osmotic protection. transport systems is similar but the substrate affinity of the monosaccharide transport system is much higher than that of the polyol transport system, Km 0.67 mM and 1.3 mM, respectively. However, one cannot exclude that the activity of MTD may be repressed by sugar as was reported in celery (Prata et al. 1997), impairing the intracellular conversion of mannitol before glucose depletion. Since mannitol transport plays a key role in source–sink interaction in O. europaea, it is likely that the expression and activity of mannitol transporters are highly regulated by mannitol levels. Our data indeed show that in O. europaea suspension-cultured cells, alterations in mannitol levels have a pronounced effect on the expression and mannitol transport activity of OeMaT. When high mannitol is present, energy-independent diffusional uptake is the preferred mode of mannitol absorption, and OeMaT expression and transport activity are maintained at basal levels. Whether non-saturable mechanisms involved in the diffusional uptake may play important roles in sink cells of olive in vivo needs further investigation, but it may be possible owing to the high sugar content in sink tissues (Patrick 1997). While non-saturable mechanisms of sugar and polyol transport were also reported in other plant cells and tissues (Delrot 1989, Keller 1991, Krook et al. 2000, Oliveira et al. 2002, Conde et al. 2006), its underlying mechanisms are still poorly understood. Several mechanisms or a combination of them could account for ‘diffusion-like’ kinetics: non-specific permeation of the sugar by free diffusion across the plasma membrane, involvement of carrier(s) or channels with very low affinity, or endocytic processes as reported by Etxeberria et al. (2005). When external mannitol decreases to residual levels, the linear transport component no longer sustains mannitol uptake at a rate sufficient to allow efficient activity of MTD, which exhibits a quite high Km of 40 mM, and the involvement of a concentrative, energy-dependent transport system becomes critical. Following mannitol depletion, OeMaT transcript levels and polyol : Hþ symport H+ mannitol H+ mannitol H+ mannitol H+ mannitol High salinity H+ mannitol NAD+ NADH H+ mannitol MTD NaCl NaCl NaCl NaCl NaCl NaCl Mannitol Mannitol NaCl Fig. 9 Mannitol NaCl H+ MTD Mannitol Mannose Low salinity H+ mannitol NaCl Regulation of mannitol transport and metabolism as a mechanism providing salt tolerance in O. europaea. NaCl NaCl NaCl Mannitol uptake in Olea europaea Indeed, after a salt pulse, a high percentage of mannitolgrown cells remained viable 24 h after addition of 250 and 500 mM NaCl, contrasting with the dramatic decrease of cell viability in sucrose-grown cells. Similarly, the growth rate of heterotrophic celery cell suspensions cultivated with sucrose was much more inhibited by NaCl than that of mannitol-grown cells, although it was demonstrated that both types of cells accumulated soluble sugars to the same osmotic potential (Pharr et al. 1995). Growing plants on soil with high salinity represents a challenge for the future, and it is therefore important to understand the strategies used by plants to cope with such stress. Given the apparent potential of mannitol for osmoprotection, the engineering of plants with mannitol metabolism is worth investigating. Although it is clear that mannitol and sorbitol are translocated in phloem from their site of synthesis to their site of use, in a way very similar to sucrose in most plants, there is some controversy about the pathways involved in phloem loading and unloading. In the case of mannitol, unloading pathways have been poorly studied, although carrier-mediated mannitol uptake had been demonstrated in tissue discs and vacuoles of storage parenchyma of celery petioles (Keller and Matile, 1989, Keller 1991, Greutert et al. 1998). In the present work, we showed that OeMaT is expressed differentially during olive fruit maturation, suggesting apoplastic unloading. This is in contrast to the initial claim that polyol-transporting species such as the olive tree were symplastic loaders (Flora and Mandore 1993). However, the co-existence of symplastic connections between the sieve tubes and olive pulp cells cannot be ruled out. Thus, mannitol transport by OeMaT1 exerts an important role in the context of olive fruit maturation. Materials and Methods Cell suspensions and growth conditions Cell suspensions of O. europaea L. var. Galega Vulgar were maintained in 250 ml flasks on a rotatory shaker at 100 r.p.m., in the dark, at 258C on modified Murashige and Skoog (MS) medium (Murashige and Skoog 1962), supplemented with 1% (w/v) mannitol, 0.5% (w/v) mannitol plus 0.5% (w/v) glucose, or 1% sucrose. Cells were subcultured weekly by transferring 10 ml aliquots into 70 ml of fresh medium. Growth was monitored as described previously (Conde et al. 2006). Transport studies of radiolabeled substrates and proton uptake Harvested cells were centrifuged, washed twice with ice-cold culture medium without sugar at pH 4.5, and resuspended in the same medium at a final concentration of 5 mg DW ml1. To estimate the initial uptake rates of D-[14C]mannitol, 1 ml of cell suspension was added to 10 ml flasks, with shaking (100 r.p.m.). After 2 min of incubation, at 258C, the reaction was started by the addition of 40 ml of an aqueous solution of radiolabeled sugar-alcohol at the desired specific activity and concentration. The specific activities were defined according to the 51 final concentration of the polyol in the reaction mixture, as follows: 500 d.p.m. nmol1 (0.1–2 mM), 100 d.p.m. nmol1 (5–20 mM). Sampling times were 0, 60 and 180 s, time periods during which the uptake was linear. Washing, radioactivity measurements and calculations were performed as described by Conde et al. (2006). Competition between labeled substrates and other sugars and polyols was tested by running competitive uptake kinetics. Inhibition of D-[1-14C]mannitol transport by non-labeled sugars and polyols was assayed by adding simultaneously the labeled and non-labeled substrate. The concentration range of labeled mannitol varied from 0.1 to 2 mM and the final concentration of the unlabeled substrate was at least 10-fold higher than the Km value estimated for the transport system. Competitive inhibition of 14 D-[U- C]glucose transport (0.02–0.5 mM) was studied with 20 mM of either mannitol, sorbitol or dulcitol. Mannitol-induced proton uptake in the cells was measured as described earlier (Conde et al. 2006). Cloning of a mannitol transporter gene To identify potential cDNA sequences encoding mannitol transporters in O. europaea, degenerated primers were designed based on conserved regions of plant polyol transporters. The primers were OeY50 [forward, 50 -TTTTAGCTTCAATGA(A/ C)TTC(A/C)-30 ] and OeY30 [reverse, 50 -CAA(C/T)TCTTTCCA CAC(A/T)GC-30 ]. RT–PCR was performed on RNA extracted from suspension-cultured cells exhibiting high mannitol transport activity. The amplified 501 bp cDNA was cloned into the pGEM-T easy vector (Promega, Madison, WI, USA) according to the manufacturer’s instructions, sequenced and subsequently named OeMaT1. RNA gel blot analysis Total RNAs from olive fruits, harvested at green, cherry and black stages of the ripening process, and from olive cell suspension samples were isolated by phenol extraction and 2 M LiCl precipitation (adapted from Howell and Hull 1978). RNA blot analysis was conducted as described in Conde et al. (2006), using a partial [32P]OeMaT1 probe. Southern blot analysis Genomic DNA of O. europaea was isolated from olive fruit according to Steenkamp et al. (1994). The DNA (10 mg) was digested with EcoRI, HindIII, XbaI and XhoI restriction enzymes. Digested genomic DNA was separated by electrophoresis in a 0.8% agarose gel, and blotted on a Hybond N membrane (Amersham, Little Chalfont, UK). The membrane was prehybridized for 3 h at 558C in 250 mM sodium phosphate buffer pH 7.2, 1% bovine serum albumin (BSA), 7% SDS, 1 mM EDTA. The membrane was hybridized overnight at 558C in the same buffer containing randomly primed [32P]OeMaT1 probe (prime-a-gene, Promega). The membrane was washed twice in 2 SSC containing 0.1% SDS for 15 min and once for 5 min in 0.1 SSC and 0.1% SDS at 558C. The membrane was exposed to an autoradiographic film and imaged using a Bio-Imaging analyzer (Bio-Rad personal molecular imager FX). Determination of cell viability FDA and PI double staining was used to estimate cell viability, as described earlier (Jones and Senft 1985). A concentrated stock solution of FDA (500 mg ml1, Sigma, St Louis, MO, USA) was prepared in dimethylsulfoxide and of PI 52 Mannitol uptake in Olea europaea (500 mg ml1, Sigma) in water. For the double staining protocol, 1 ml of cell suspensions was incubated with 10 ml of FDA stock solution and 1 ml of PI stock solution in the dark for 10 min at room temperature. Cells were observed under a Leitz Laborlux S epifluorescence microscope with a 50 W mercury lamp and appropriate filter settings. Images were acquired with a 3CCD color video camera (Sony, DXC-9100P), a frame grabber (IMAGRAPH, IMASCAN/Chroma P) and software for image management and archiving (AxioVision Version 3.0, Carl Zeiss Vision, GmbH). Determination of mannitol dehydrogenase activity Protein extraction and MTD activity assays were determined as described by Stoop and Pharr (1993). Olea europaea suspensioncultured cells were harvested as described above and ground in a chilled mortar using a 1 : 4 (v/v) powder : buffer ratio. The extraction buffer contained 50 mM MOPS (pH 7.5), 5 mM MgCl2, 1 mM EDTA, 5 mM dithiothreitol (DTT), 1 mM phenylmethylsulfonyl fluoride (PMSF) and 1% (v/v) Triton X-100. Homogenates were centrifuged at 20,000g for 20 min and the supernatants were stored on ice. MTD activity was assayed by monitoring the reduction of NADþ spectrophotometrically at 340 nm. Assays were conducted at room temperature (258C) in a total volume of 1 ml. The reaction mixture contained 100 mM Bis-Tris propane (pH 9.0), 2 mM NADþ, enzyme extract, and D-mannitol at the desired final concentration. The reactions were initiated by the addition of mannitol. Protein concentrations were determined by the method of Bradford (1976) using BSA as a standard. Acknowledgments This work was supported in part by the Pessoa Program (GRICES/EGIDE), the Conférence des Présidents d’Université (CPU), the Conselho de Reitores das Universidades Portuguesas (CRUP), the Fundação para a Ciência e a Tecnologia (research project no. POCI/AGR/56378/2004; grant no. SFRH/BD/10689/ 2002 to C.C.; grant no. SFRH/BD/13460/2003 to P.S.; and grant no. SFRH/PBD/17166/2004 to A.A.). References Azevedo, H., Conde, C., Gerós, H. and Tavares, R.M. (2006) The non-host pathogen Botrytis cinerea enhances glucose transport in Pinus pinaster suspension-cultured cells. Plant Cell Physiol. 47: 290–298. Bradford, M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 72: 248–254. Cakir, B., Agasse, A., Gaillard, C., Saumonneau, A., Delrot, S. and Atanassova, R. (2003) A grape ASR protein involved in sugar and abscisic acid signaling. Plant Cell 15: 2165–2180. Chauhan, S., Forsthoefel, N., Ran, Y., Quigley, F., Nelson, D.E. and Bohnert, H.J. (2000) Naþ/myo-inositol symporters and Naþ/Hþ-antiport in Mesembryanthemum crystallinum. Plant J. 24: 511–522. Conde, C., Agasse, A., Glissant, D., Tavares, R.M., Gerós, H. and Delrot, S. (2006) Pathways of glucose regulation of monosaccharide transport in grape cells. Plant Physiol. 141: 1563–1577. Drossopoulos, J.B. and Niavis, C.A. (1988) Seasonal changes of the metabolites in the leaves, bark and xylem tissues of olive tree (Olea europaea L.). 2. Carbohydrates. Ann. Bot. 62: 321–327. Delrot, S. (1989) Loading of photoassimilates. In Transport of Photoassimilates. Edited by Baker, D.A. and Milburn, J.A. pp. 167–205. Longman Scientific, Harlow, UK. Ehness, R., Ecker, M., Godt, D.E. and Roitsch, T. (1997) Glucose and stress independently regulate source and sink metabolism and defense mechanisms via signal transduction pathways involving protein phosphorylation. Plant Cell. 9: 1825–1841. Etxeberria, E., Gonzalez, P., Tomlinson, P. and Pozueta-Romero, J. (2005) Existence of two parallel mechanisms for glucose uptake in heterotrophic plant cells. J. Exp. Bot. 56: 1905–1912. Everard, J.D., Gucci, R., Kann, S.C., Flore, J.A. and Loescher, W.H. (1994) Gas-exchange and carbon partitioning in the leaves of celery (Apium graveolens L.) at various levels of root-zone salinity. Plant Physiol. 106: 281–292. Flora, L.F. and Madore, M.A. (1993) Stachyose and mannitol transport in olive (Olea europea L.). Planta. 189: 484–490. Gao, Z., Maurousset, L., Lemoine, R., Yoo, S.D., Van Nocker, S. and Loescher, W. (2003) Cloning, expression, and characterization of sorbitol transporters from developing sour cherry fruit and leaf sink tissues. Plant Physiol. 131: 1566–1575. Greutert, H., Martinoia, E. and Keller, F. (1998) Mannitol transport by vacuoles of storage parenchyma of celery petioles operates by facilitated diffusion. J. Plant Physiol. 153: 91–96. Guicherd, P., Peltier, J.P., Gout, E., Bligny, R. and Marigo, G. (1997) Osmotic adjustment in Fraxinus excelsior L.: malate and mannitol accumulation in leaves under drought conditions. Trees 11: 155–161. Howell, S.H. and Hull, R. (1978) Replication of cauliflower mosaic virus and transcription of its genome in turnip leaf protoplasts. Virology 86: 468–481. Hu, L., Lu, H., Liu, Q., Chen, X. and Jiang, X. (2005) Overexpression of mtlD gene in transgenic Populus tomentosa improves salt tolerance through accumulation of mannitol. Tree Physiol. 25: 1273–1281. Jennings, D.B., Ehrenshaft, M., Pharr, D.M. and Williamson, J.D. (1998) Roles for mannitol and mannitol dehydrogenase in active oxygen-mediated plant defense. Proc. Natl Acad. Sci. USA 95: 15129–15133. Jones, K.H. and Senft, J.A. (1985) An improved method to determine cell viability by simultaneous staining with fluorescein diacetate–propidium iodide. J. Histochem. Cytochem. 33: 77–79. Keller, F. (1991) Carbohydrate transport in disks of storage parenchyma of celery petioles. 2. Uptake of mannitol. New Phytol. 117: 423–429. Keller, F. and Matile, P. (1989) Storage of sugars and mannitol in petioles of celery leaves. New Phytol. 113: 291–299. Klepek, Y.S., Geiger, D., Stadler, R., Klebl, F., Landouar-Arsivaud, L., Lemoine, R., Hedrich, R. and Sauer, N. (2005) Arabidopsis POLYOL TRANSPORTER5, a new member of the monosaccharide transporterlike superfamily, mediates Hþ-symport of numerous substrates, including myo-inositol, glycerol, and ribose. Plant Cell. 17: 204–218. Krook, J., Vreugdehil, D. and van der Plas, L.H.W. (2000) Uptake and phosphorylation of glucose and fructose in Daucus carota cell suspensions are differentialy regulated. Plant Physiol. Biochem. 38: 603–612. Lewitt, J. (1980) Responses of Plants to Environmental Stresses. Vol II. Water, Radiation, Salt, and Other Stresses. Academic Press Inc., New York. Marsilio, V., Campestre, C., Lanza, B. and De Angelis, M. (2001) Sugar and polyol compositions of some European olive fruit varieties (Olea europaea L.) suitable for table olive purposes. Food Chem. 72: 485–490. Murashige, T. and Skoog, F. (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15: 473–497. Nelson, D.E., Koukoumanos, M. and Bohnert, H.J. (1999) Myoinositol-dependent sodium uptake in ice plant. Plant Physiol. 119: 165–172. Noiraud, N., Delrot, S. and Lemoine, R. (2000) The sucrose transporter of celery. Identification and expression during salt stress. Plant Physiol. 122: 1447–1456. Noiraud, N., Maurousset, L. and Lemoine, R. (2001a) Identification of a mannitol transporter, AgMaT1, in celery phloem. Plant Cell. 13: 695–705. Noiraud, N., Maurousset, L. and Lemoine, R. (2001b) Transport of polyols in higher plants. Plant Physiol. Biochem. 39: 717–728. Mannitol uptake in Olea europaea Oliveira, J., Tavares, R.M. and Gerós, H. (2002) Utilization and transport of glucose in Olea europaea cell suspensions. Plant Cell Physiol. 43: 1510–1517. Patonnier, M., Peltier, J. and Marigo, G. (1999) Drought-induced increase in xylem malate and mannitol concentrations and closure of Fraxinus excelsior L. stomata. J. Exp. Bot. 50: 1223–1231. Patrick, J.W. (1997) Phloem unloading: sieve element unloading and postsieve element transport. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48: 191–222. Pharr, D.M., Stoop, J.M.H., Studer Feusi, M.E., Williamson, J.D., Massel, M.O. and Conkling, M.A. (1995) Mannitol catabolism in plant sink tisues. Curr. Top. Plant Physiol. 13: 180–194. Prata, R.T.N., Williamson, J.D., Conkling, M.A. and Pharr, D.M. (1997) Sugar repression of mannitol dehydrogenase activity in celery cells. Plant Physiol. 114: 307–314. Ramsperger-Gleixner, M., Geiger, D., Hedrich, R. and Sauer, N. (2004) Differential expression of sucrose transporter and polyol transporter genes during maturation of common plantain companion cells. Plant Physiol. 134: 147–160. Roitsch, T. and Tanner, W. (1994) Expression of a sugar-transporter gene family in a photoautotrophic suspension-culture of Chenopodium rubrum L. Planta 193: 365–371. Rugini, E. and Fedeli, E. (1990) Olive as an oilseed crop. In Biotechnology in Agriculture and Forestry. Legumes and Oilseed Crops. Edited by Bajaj, Y.P.S. pp. 593–641. Springer-Verlag, Berlin, Germany. Salmon, S., Lemoine, R., Jamai, A., Bouchepillon, S. and Fromont, J.C. (1995) Study of sucrose and mannitol transport in plasma-membrane vesicles from phloem and nonphloem tissues of celery (Apium graveolens L.) petioles. Planta 197: 76–83. Schneider, S., Schneidereit, A., Konrad, K.R., Hajirezaei, M.R., Gramann, M., Hedrich, R. and Sauer, N. (2006) Arabidopsis INOSITOL TRANSPORTER4 mediates high affinity Hþ-symport of myo-inositol across the plasma membrane. Plant Physiol. 141: 565–577. Smirnoff, N. and Cumbers, Q. (1989) Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 28: 1057–1060. 53 Steenkamp, J., Wiid, I., Lourens, A. and Vanhelden, P. (1994) Improved method for DNA extraction from Vitis vinifera. Am. J. Enol. Viticult. 45: 102–106. Stoop, J.M.H. and Pharr, D.M. (1993) Effect of different carbon sources on relative growth rate, internal carbohydrates, and mannitol 1-oxidoreductase activity in celery suspension cultures. Plant Physiol. 103: 1001–1008. Stoop, J.M.H. and Pharr, D.M. (1994) Mannitol metabolism in celery stressed by excess macronutrients. Plant Physiol. 106: 503–511. Stoop, J.M.H., Williamson, J.D. and Pharr, D.M. (1996) Mannitol metabolism in plants: a method for coping with stress. Trends Plant Sci. 5: 139–144. Tarczynski, M.C., Jensen, R.G. and Bohnert, H.J. (1993) Stress protection of transgenic tobacco by production of the osmolyte mannitol. Science 259: 508–510. Tattini, M., Ponzio, C., Coradeschi, M.A., Tafani, R. and Traversi, M.L. (1994) Mechanisms of salt tolerance in olive plants. Acta Horticult. 356: 181–184. Therios, I.N. and Misopolinos, N.D. (1988) Genotypic response to sodiumchloride salinity of 4 major olive cultivars (Olea europaea L.). Plant Soil 106: 105–111. Thomas, J. C., Sepahi, M., Arendall, B. and Bohnert, H.J. (1995) Enhancement of seed germination in high salinity by engineering mannitol expression in Arabidopsis thaliana. Plant Cell Environ. 18: 801–806. Vitagliano, C. and Sebastiani, L. (2002) Physiological and biochemical remarks on environmental stress in olive (Olea europaea L.). Acta Horticult. 586: 435–440. Watari, J., Kobae, Y., Yamaki, S., Yamada, K., Toyofuku, K., Tabuchi, T. and Shiratake, K. (2004) Identification of sorbitol transporters expressed in the phloem of apple source leaves. Plant Cell Physiol. 45: 1032–1041. Williamson, J.D., Stoop, J.M.H., Massel, M.O., Conkling, M.A. and Pharr, D.M. (1995) Sequence analysis of a mannitol dehydrogenase cDNA from plants reveals a function for the pathogenesis-related protein ELI3. Proc. Natl Acad. Sci. USA 92: 7148–7152. (Received September 12, 2006; Accepted November 12, 2006)