* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Fulltext PDF - Indian Academy of Sciences
No-SCAR (Scarless Cas9 Assisted Recombineering) Genome Editing wikipedia , lookup
Gene expression programming wikipedia , lookup
Quantitative trait locus wikipedia , lookup
Genome (book) wikipedia , lookup
Behavioural genetics wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Genetic drift wikipedia , lookup
Epigenetics of human development wikipedia , lookup
Koinophilia wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
Oncogenomics wikipedia , lookup
Preimplantation genetic diagnosis wikipedia , lookup
Polymorphism (biology) wikipedia , lookup
Skewed X-inactivation wikipedia , lookup
Y chromosome wikipedia , lookup
Neocentromere wikipedia , lookup
Point mutation wikipedia , lookup
Population genetics wikipedia , lookup
X-inactivation wikipedia , lookup
Human leukocyte antigen wikipedia , lookup
Genomic imprinting wikipedia , lookup
Microevolution wikipedia , lookup
Medical genetics wikipedia , lookup
Hardy–Weinberg principle wikipedia , lookup
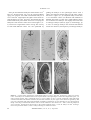
Indian Academy of Sciences Effects of mutations at the stambh A locus of Drosophila melanogaster M. KUMAR, MINU JOSEPH and SHANTI CHANDRASHEKARAN* Division of Genetics, Indian Agricultural Research Institute, New Delhi 110 012, India Abstract We report novel findings on the cytogenetic location, functional complexity and maternal and germline roles of the stambh A locus of Drosophila melanogaster. stmA is localized to polytene bands 44D1.2 on 2R. stmA mutations are of two types: temperature-sensitive (ts) adult and larval paralytic or unconditional embryonic or larval lethal. Twelve alleles reported in this study fall into two intragenic complementing groups suggesting that stmA is a complex locus with more than one functional domain. Some unconditional embryonic lethal alleles show a ‘neurogenic’ phenotype of cuticle loss accompanied by neural hypertrophy. It is shown that embryos of ts paralytic alleles also show mild neural hypertrophy at permissive temperatures while short exposure to heat induces severe cuticle loss in these embryos. stmA exerts a maternal influence over heat-induced cuticle loss. Unconditional embryonic lethal alleles of stmA are also germline lethal. [Kumar M., Joseph M. and Chandrashekaran S. 2001 Effects of mutations at the stambh A locus of Drosophila melanogaster. J. Genet. 80, 83–95] Introduction The mutation stambh A (stmA) (2–56.8 cM) in Drosophila melanogaster was discovered through its phenotype of reversible temperature-sensitive (ts) paralysis in our laboratory (Shyngle and Sharma 1985). Adults and larvae homozygous for stmA1 and stmA2 are paralysed within 2–4 minutes of exposure to 38ºC temperature. When they are brought back to 23–24ºC the flies recover to normal behaviour within 10–12 minutes (Chandrashekaran and Sarla 1993). Mutations isolated later included several unconditional multiphasic lethal alleles (Kumar 1993). Some of them were embryonic lethals and showed pronounced hypertrophy of the embryonic dorsal neurogenic region and mild hypertrophy of the ventral neurogenic regions (Chandrashekaran and Sarla 1993). Neural hypertrophy was accompanied by hypotrophy of the cuticle, closely resembling the phenotype of mutations in genes of the Notch-Delta category collectively called neurogenic genes (Lehmann et al. 1983). Adults homo- *For correspondence. Email: [email protected]. Keywords. zygous for stmA are also resistant to the neuropoison veratridine (Chandrashekaran 1993) which causes lethality by binding to a protein subunit of the voltagegated transmembrane sodium channels of nerve membranes (Catterall 1986). It is unlikely that veratridine resistance in mutant flies is due to direct involvement of stmA in the structure or function of the transmembrane sodium channels. Reasons for doubt arise from the observation that double mutants of stmA and para, a structural gene for neuronal voltage-gated sodium channels in Drosophila (Loughney et al. 1989), did not show any phenotypic interaction (Chandrashekaran and Sarla 1993). Currently available information on the phenotypic effects of mutations at stmA points to its role in neural development. However, little else is known about the genetics, molecular nature and other developmental functions of stmA. In this paper, we report several new findings that (i) demonstrate the complex nature of the stmA locus, (ii) provide further evidence for the role of stmA in neuroectodermal development, and (iii) demonstrate the maternal and germline function of stmA. As a prelude to its cloning and molecular analysis the precise Drosophila melanogaster; stambh A; maternal effect; cuticle loss. Journal of Genetics, Vol. 80, No. 2, August 2001 83 M. Kumar et al. cytological location of stmA and transposon tagging are also reported in this study. Material and methods Fly maintenance: Flies were grown on standard corn meal, yeast and unrefined sugar media at 23–24ºC unless stated otherwise. removed by replacing the X and 3rd chromosomes by non-P-bearing M-5 and TM6 balancers. The second chromosome was outcrossed with a P-element-free, multiple-marked stock al dp b pr c px sp. Recombinant chromosomes carrying stmAP with al dp b pr were recovered in the first step. In a second recombination experiment the chromosomal region distal to stmA was replaced with markers c px sp. This process almost made sure that the new P-induced allele of stmA did not carry any other P elements. Fly stocks stmA alleles: All stmA alleles originated from our own experiments (Shyngle and Sharma 1985; Chandrashekaran and Sarla 1993; present study). Birm-2 and Jumpstarter: Both Birm-2 and Jumpstarter (JS) stocks were kindly gifted by Dr William Engels. The Birm-2 genotype carries ~ 17 natural but defective nonautonomous P elements on its second chromosome (Robertson et al. 1988). JS is a third chromosome carrying the P transposon ∆2-3, a stable source of transposase. The JS stock used in this study is CyO / Sp; ∆2-3 Sb / TM6B. Deficiencies: The deficiencies on chromosome 2R used in this study are listed in table 6. + D1 D ovoD1: P[w ovo ] / Ms(2)bw / CyO carries the dominant female sterile (DFS) mutation ovoD1 on chromosome 2R at 55E (Chou et al. 1993). Isolation of alleles: New alleles were identified on the basis of noncomplementation with stmA2 for ts paralysis in the F1 or with stmA12 for lethality in F2. F2 noncomplementation for lethality over stmA12: Male flies of the genotype al dp b pr or Canton S were irradiated with gamma rays (30 gray; from a 60Co source) and mated to Cy / Gla females. Cy or Gla male progeny were individually mated to stmA12 / Pm virgins. The F2 progeny of each vial was scored for the absence of nonbalancer flies and putative lethal alleles were recovered over Pm. Temperature-induced paralysis: Adult flies were placed in thin-walled glass vials and held in a water bath at the desired temperature. Flies that had fallen on their back were taken as paralysed. Scoring embryonic cuticle loss phenotypes: Cleared cuticle preparations were made according to van der Meer (1977). The cleared embryos were grouped into the following five classes based on increasing loss of continuous cuticle: class I, normal looking embryos with continuous cuticle and head skeleton; class II, embryos with shrunken cuticle, large dorsal hole overlying the brain, malformed head, germband retraction often incomplete; class III, embryos with large dorsal and ventral cuticle holes, head skeletal structures faintly visible; class IV, only a narrow strip of cuticle visible; class V, no continuous cuticle. F1 noncomplementation for ts paralysis over stmA2 EMS induced alleles: Male flies (Canton-S) were fed 0.3% 2 EMS and mated en masse to b stmA virgins. The F1 males were tested for paralysis at 38ºC followed by recovery at 23–24ºC. The putative b+ stmA? / b stmA2 males were outcrossed to Cy / Pm balancer-bearing females and the new stmA-allele-bearing chromosomes recovered over Pm (recognized as black-bodied flies because the Pm balancer carries the marker b) and intercrossed after retesting to confirm allelism. Hybrid dysgenesis induced alleles: Hybrid dysgenic + / Y; Birm-2 / CyO; ∆2-3 Sb / + males were mated to b stmA1 virgins and the progeny reared at 18ºC. Non-Cy non-Sb male progeny were tested for paralysis at 38ºC and recovery at 23ºC. Paralysis was confirmed over b stmA1 in the following generation and confirmed lines were extracted against Pm. Any P elements that might have lodged themselves on chromosomes X and 3 were 84 Benzer’s countercurrent test: The countercurrent distribution procedure of Benzer (1967) was used to score the negative geotactic response of 3–4-day old adult males at 23–24ºC. Twenty males per test and 60 per genotype were used. Flies were adapted to ambient light in test tubes for 15 min and distributed by countercurrent for their ability to climb up the walls of the glass tube (negative geotactic response) in five cycles of 60 sec duration. The negative geotactic response was calculated as: (n1 × 0) + (n2 × 1) + (n3 × 2) + (n4 × 3) + (n5 × 4) + (n6 × 5)]/N, where n1, n2, n3, . . . n6 denote the numbers of flies in tubes 1 . . . 6 and N = n, the total number of flies. Immunostaining of the embryonic nervous system: The nervous system was stained with the neuronal-specific mouse antibody Mab 22C10. Briefly: Dechorionated eggs were fixed in 4% paraformaldehyde in heptane and devitellinized in heptane–methanol. Embryos were per- Journal of Genetics, Vol. 80, No. 2, August 2001 Mutations at stambh A in Drosophila melanogaster meabilized with 0.1% Triton X-100 in phosphate-buffered saline (PBS). The embryos were incubated in primary antibody (dilution 1 : 50) overnight 4ºC. The secondary antibody was biotinylated anti–mouse (dilution 1 : 200). The antibody binding was visualized by horseradish peroxidase activity (dark brown-black color) using diaminobenzidine (5 mg per 2 ml in PBS with 0.1% Tween 20). Female germline clones: Clones were induced by irradiating larvae from the cross stmA + / SM5 × SM5 / + [Pw+ ovoD] with gamma rays (1 gray) from a 60Co source. The [Pw+ ovoD] chromosome carries the dominant X-linked female-sterile mutation ovoD at 55DE on chromosome 2R (Chou et al. 1993). All stmA + / + [Pw+ ovoD] females were mated to wild-type males and tested for egg laying. Results Alleles Four independent experiments were conducted to screen for new stmA alleles (table 1) and 19 alleles were recovered among approximately 70,000 chromosomes tested. Of these, eight alleles have been lost and only 11 new alleles are being maintained in our collection. Eight out of the 11 remaining alleles are unconditional lethals and three are homozygous viable. Adults and larvae homozygous for these three viable stmA alleles also get paralysed at 38ºC like the original stmA1 (table 2). stmA larvae also are paralysed in about 2–4 minutes (depending upon the allele), stretch out and lose all muscle tone on paralysis. The eight unconditional lethal alleles showed a partially dominant lethal phenotype with viability ranging from 30% to 50%. Lethality was multiphasic and spanned embryonic, larval and pupal stages (data not shown). Table 1. Flies homozygous for the alleles stmA1, stmA2, stmAP1 and stmAP4 were paralysed within 2–3 minutes at 38ºC, while heterozygous stmA1 / +, stmA2 / + and stmAP4 / + flies were paralysed after a 17–24-minute exposure at 38ºC (table 2). For wild-type (Canton-S) males maintained in our laboratory, it takes 32 minutes for paralysis at 38ºC. The paralytic behaviours of wild-type, stmA / stmA and stmA / + flies differ greatly. Homozygous stmA adults show a gradual slowing down of physical activity within 30–45 seconds of heat exposure before they are completely immobilized and fall on their backs. Canton-S flies remain mobile even till one minute before paralysis sets in. After they are reverted to 23ºC the paralysed Canton-S flies revive to normal behaviour in 30 seconds while stmA flies take over 10 minutes to begin moving (and more than 24 hours before they resume completely normal mobility, preening, feeding and mating behaviour). The paralytic behaviour of stmA / + heterozygotes is very similar to Canton-S flies in the first half of their exposure to high temperature, after which their physical activity slows down. Unlike Canton-S flies, which revive in 30 seconds, stmA / + heterozygotes take up to 3–4 minutes to revive. None of the unconditional lethals showed dominant paralytic phenotypes at 38ºC. stmA adults show abnormal (hyperactive or hypoactive) behaviour at temperatures of 23–24ºC (a temperature which is otherwise defined as permissive for the paralytic expression). stmA1 and stmAP1 adults are hypoactive and stmA2 hyperactive. To quantify these differences in behaviour the negative geotactic responses of adult males were measured by Benzer’s countercurrent test. Canton-S flies, if tapped to the bottom of a glass tube, respond by climbing up the walls (a negative geotactic response). Negative geotactic response scores of adult Canton-S males was 2.26. stmA1, stmAP1 and stmP4 adults were weakly negatively geotactic while stmA2 adults were highly negatively geotactic (table 3). This difference in the negative geotactic responses of the various stmA alleles is also reflected in routine physical activity in that Summary of stmA allele search experiments. No. of alleles Expt Mutagen 1. EMS 2. 3. 4. P element EMS Gamma rays Screening criterion Chromosomes screened Unconditional lethal Conditional paralytic Paralysis at 38ºC over stmA1 and recovery at 24ºC Same as above Same as above Lethality over stmA12 39,000 2 1 2, 7, 12a 7827 17,280 6865 0 2 0 5 4 5 P1, P2, P3, P4, P5b KP1, KP2, . . ., KP6c 1-2, 1-4, 2-2, 18-2, 27-1d Alleles a Chandrashekaran and Sarla (1993). P2, P3, P5 lost. c KP1, KP3, KP5, KP6 lost. d 2-2 lost. b Journal of Genetics, Vol. 80, No. 2, August 2001 85 M. Kumar et al. stmA2 (strongly negatively geotactic) adults are very hyperactive while adults of stmA1, stmAP1 and stmAP4 (weakly negatively geotactic) are sluggish and almost always flightless. Complementation On the basis of intragenic complementation among the four ts paralytic and eight unconditional lethals (table 4, figure 1) three classes of alleles could be defined. The lethal alleles (7, 12, 18-2, 1-2 and KP2) comprise group I, which do not complement members of groups II and III. Group II members 27-1 and 1-4 (both unconditional lethal) complement members of group III (KP4, 1, 2, P1 and P4, comprising unconditional lethal and viable ts paralytic). Table 2. Time taken (minutes; seconds) for 100% paralysis of adult males of various heteroallelic combinations of stmA ts paralytic alleles at a temperature of 38ºC. + 1 2 P1 P4 + 1 2 P1 P4 32;00 20;00 4;00 17;30 3;00 3;00 32;00 2;00 2;00 2;00 24;00 3;00 3;00 2;00 3;00 Table 3. Negative geotactic responses of stmA and wild-type adults measured by Benzer’s countercurrent test. Allele + 1 2 P1 P4 Response score Time (min; sec) taken for 100% paralysis of adults at 38ºC 2.26 1.26 4.12 0.3 1.6 32;00 4;00 3;00 2;00 3;00 It was previously reported that mutations leading to ts paralysis were partial gain-of-function neomorphs, while unconditional lethals were partial (hypomorph) or complete loss-of-function amorphs. This conclusion was based on the observation that ts paralytic / lethal trans heterozygous adults showed weaker paralysis than their respective ts paralytic homozygotes (Chandrashekaran and Sarla 1993). The paralytic phenotypes of the 32 possible viable / lethal heteroallelic and four homoallelic combinations of stmA are shown in table 5. Compared to their respective homozygotes, 11 heterozygotes were weaker, eight were stronger and five were identical in their ts paralytic phenotypes. Eight trans heterozygotes showed complementation (wild-type phenotype) for the ts paralytic effect. Absence of any straightforward relation between paralytic phenotypes of paralytic / lethal heterozygotes and their respective homozygotes indicates that stmA is not a simple locus but very likely consists of more than one functional domain, mutations in which lead to ts paralysis or lethality or both. Cytological mapping A previous study (Chandrashekaran and Sarla 1993) revealed that flies of the genotype stmA1 / stmA1; Tp2; 3 P32 (containing two copies of stmA1 and a wild-type copy of the 2nd chromosome region 41A to 44D4.8 on chromosome 3) showed wild type behaviour. stmA was therefore roughly mapped to the 41A–44D4.8 region. All deletions spanning 41A through 41C and 42A16 through 43F8.9 were wild type over stmA. The stmA12-bearing chromosome was associated with a visible cytological deficiency 42A8–42B2 which could not be separated by recombination from the stmA lethal at the time of the study. On that basis it was concluded that stmA mapped to the 42A16–42B2 segment. Deficiencies distal to 43F8.9 up to 44D4.8 were not tested because of the ‘tight’ association of the 42A8–42B2 deficiency with stmA12. However, subsequently we were able to separate the lethal stmA12 allele from the deficiency, making it essential to Table 4. Interallelic complementation among 12 alleles of stmA. Alleles 1, 2, P1 and P4 are conditional paralytic alleles while the rest are unconditional lethal alleles. ‘+’ indicates a wild-type phenotype, ‘-’ indicates ts paralysis or unconditional lethality. 1 2 P1 P4 1–2 1–4 7 12 18–2 27–1 KP2 86 2 P1 P4 1–2 1–4 7 12 18–2 27–1 KP2 KP4 – – – – – – – – – – + + + + – – – – – – – – – – – – – – – – – – – – – – + + + + – – – – – – – – – – – – – – – – – – – – + – – – + – Journal of Genetics, Vol. 80, No. 2, August 2001 Mutations at stambh A in Drosophila melanogaster redetermine the cytological location of stmA. In this study five new deficiencies have been analysed (table 6), all with at least one breakpoint distal to 43F8.9, which was the distalmost breakpoint in the previous study. Among the five deficiencies tested, two were overlapping: Df(2R)H3C1 [43F to 44D3.8] and Df(2R)H3E1 [44D1.4 to 44F12], both of which uncovered the 12 ts paralytic and lethal stmA alleles. stmA therefore maps to the overlapping 44D1.4 to 44D3.8 region stretching from the proximal breakpoint 44D1.4 of Df(2R)H3E1 to the distal breakpoint 44D3.8 of Df(2R)H3C1 (figure 2). The breakpoints of deficiency Df(2R)44CE, according to Flybase (http://www.flybase.bio.indiana.edu), are 44C1.2 (proximal) and 44E1.4 (distal). It is therefore deficient for the relevant 44D1.4 to 44D3.8 region that is common to Df(2R)H3C1 [43F to 44D3.8] and Df(2R)H3E1 [44D1.4 to 44F12]. Df(2R)44CE is therefore expected to uncover the mutant phenotype of stmA. However, Df(2R)44CE / stmA flies were surprisingly wild type. It can therefore be concluded that the 44C1.2; 44E1.4 breakpoints of Df(2R)44CE were probably determined incorrectly. A careful study of polytene chromosomes of Df(2R)44CE / + larvae shows (figure 2b) that our premise was indeed correct. Df(2R)44CE is not one continuous deficiency but is made up of two adjacent deficiencies, one small deficiency for the two bands 44C1.2 and another for the bands 44D3 to 44E1.4. The polytene band 44D1.2 is clearly present (see figure 2b) in between the two adjacent deficiencies. 44D1.2 is the only band that is deficient in Df(2R)H3C1 and Df(2R)H3E1 but is present in Df(2R)44CE. On the basis of the noninclusion of stmA in Df(2R)44CE and its inclusion in Df(2R)H3C1 and Df(2R)H3E1, stmA is placed in the polytene bands 44D1.2 (figure 2a). Heat-induced embryonic lethality and cuticle loss in the ts paralytic alleles of stmA Figure 1. Diagrammatic representation of complementation among the stmA alleles. It was reported earlier (Chandrashekaran and Sarla 1993) that the unconditional lethal alleles stmA12 and stmA7 show dorsal embryonic cuticle loss overlying the brain Table 5. Interalleleic complementation among eight unconditional lethal alleles and four ts paralytic alleles of stmA. ‘–’ indicates ts paralysis and figures in parenthesis are time taken (min;sec) for 100% paralysis of adult males at 38ºC, ‘+’ indicates a wild-type phenotype defined as absence of paralysis for up to 32 minutes at 38ºC. Homozygote 1–2 1–4 18–2 27–1 KP2 KP4 7 12 1 4;00 + 3;00 P1 2;00 P4 3;00 – (4;30) – (2;00) – (3;00) – (3;00) + 2 – (5;00) – (3;00) – (3;00) – (3;00) – (1;40) – (2;10) – (2;45) – (2;45) – (2;30) – (2;00) – (2;00) – (3;00) – (12;00) – (4;00) – (2;15) – (2;20) – (23;00) – (4;00) – (2;15) – (2;20) Table 6. + + + + + + List of deficiencies on chromosome arm 2R used to map stmA. Breakpoints S. No. 1 2 3 4 5 Deficiency (2R) cn 87e cn 9 H3C1 44CE H3E1 Bloomington stock numbers 224 3368 198 3643 201 Proximal Distal Whether stmA+ 42B4.C1 42E 43F 44C1.2* 44D1.4 43F.44A1 44C 44D3.8 44E2.4* 44F12 Yes Yes No Yes No *Breakpoints as published in http://www.flybase.bio.indiana.edu. In this study deficiency 44CE is shown to consist of two deficiencies: (i) for the doublet band 44C1.2 and (ii) for bands 44D3 to 44E1.4. Journal of Genetics, Vol. 80, No. 2, August 2001 87 M. Kumar et al. coupled with neural hypertrophy of the dorsal brain lobes and the ventral neurogenic region. Since adults of the ts paralytic alleles stmA1 and stmA2 show abnormal behaviour at 23ºC and at 38ºC it is reasonable to expect structural anomalies in the nervous system of embryos, larvae and adults. Also, stmA embryos rarely survived a even brief accidental exposure to temperatures of 30ºC and above. The present study revealed that 18-hour-old stmA1 embryos (reared at 23ºC) show a mild hypertrophy of the embryonic brain lobes and ventral nerve chord (figure 3, b, d, f). Gross structural abnormalities in neuronal connections or cuticle loss were not observed. H3E1 44CE H3C1 b Figure 2. a, Polytene chromosome map of section 44 of chromosome 2 right arm (electron micrographic and Bridges’ 1935 map), showing the position of stmA (short thick line) with respect to the breakpoints of Df(2R)H3C1 and Df(2R)H3E1 and the corrected breakpoints of Df(2R)44CE. Dark solid lines and arrows show the extent of the deficiency; dashed line with arrowhead shows the breakpoint uncertainty. b, Polytene chromosomes of Df(2R)44CE / + larvae showing polytene band 44D1.2 (small arrow) in between the two adjacent deficiencies 44C1.2 (two-headed dotted arrow) and 44D3 to 44E1.4 (two-headed bold arrow). 88 Journal of Genetics, Vol. 80, No. 2, August 2001 Mutations at stambh A in Drosophila melanogaster Given that stmA1 embryos show mild neural hypertrophy at 23ºC, it is pertinent to ask if mild expression will be exaggerated if stmA1 embryos are exposed to higher temperature. Morphological segregation of neuroblasts from epidermoblasts occurs during stages 8–10 (soon after three hours from egg laying at 24ºC after completion of the ventral furrow) of embryogenesis (Campos-Ortega 1993). The embryos of stmA1, stmA2 and Canton-S were exposed to 31, 33 and 35ºC at two stages, i.e. syncitial blastoderm (0–0.5 h) and cellular blastoderm (3 h), prior to overt segregation of neuroblasts from the neuroectoderm. In general, stmA1 and stmA2 embryos were much more sensitive to heat treatment than the wild-type embryos and showed high levels of heatinduced lethality (table 7). Three-hour-old embryos were more tolerant to heat shock than freshly laid eggs. Genotypic differences between stmA and Canton-S were distinct at 33ºC where the mutants showed 3 5-fold higher lethality than the wild type. Exposure to 35ºC temperature caused nonspecific embryonic lethality, especially in the eggs at 0 h, and was not considered for further analysis. Figure 3. Embryonic nervous system of 18-hour-old (stage 15) wild-type (a, c, e) and stmA1 (b, d, f) embryos reared at 23°C. Note the enlarged and denser antennomaxillary nerve complex (amc), ventral nerve chord (vnc) and brain (b) lobes in stmA. All embryos are oriented with anterior to the top. Photos a, e, b and f are ventral views, and c and d are lateral views. Embryos were stained with monoclonal antibody 22C10. Magnification: a, b, c, d, 16×; e, 64×; f, 32×. Table 7. Heat-induced lethality in wild-type and stmA embryos. Temperature 31°C Age of embryo (hAEL) 33°C Age of embryo (hAEL) 35°C Age of embryo (hAEL) Genotype 0 % EL (N) 3 % EL (N) 0 % EL (N) 3 % EL (N) 0 % EL (N) 3 % EL (N) + stm A1 stm A2 15.5 (543) 6.9 (302) 24.2 (749) 7.3 (425) 1.4 (419) 11.9 (370) 18.7 (502) 53.3 (272) 41.7 (511) 8.7 (172) 43.3 (172) 45.1 (359) 87.5 (200) 93.0 (192) 95.0 (80) 28.0 (205) 88.4 (190) 90.0 (270) hAEL, Hours after egg laying; %EL, per cent embryonic lethality; N, number of embryos scored. Journal of Genetics, Vol. 80, No. 2, August 2001 89 M. Kumar et al. Since the unconditional embryonic lethal alleles stmA12 and stm7 showed cuticle loss over the neuroectodermal region, the question was asked if heat-killed embryos of stmA1 and stmA2 might mimic this effect. Observations on cleared embryos of stmA1 and stmA2 showed that they did indeed suffer from pronounced cuticle loss overlying the embryonic brain and the ventral nerve chord (figure 4, b– f). Cuticle loss ranged from weak to strong. The severity of heat-shock-induced cuticle loss was quantified by grading the embryos in five phenotypic classes: class I (figure 4a) had near-normal embryos with intact cuticles and class V (figure 4, e, f) comprised embryos with little or no continuous cuticle (see Material and methods for detailed description of each class). Heat-induced cuticle loss in mutant stmA embryos was roughly three times that in wild-type embryos (table 8, figure 5). Interestingly, up to 35% of wild-type embryos also showed heat-induced cuticle loss. However, the frequency of wild-type embryos Figure 4. Cleared cuticle preparations of heat-killed embryos of stmA1 and stmA2 belonging to cuticle loss classes I to V. All embryos are oriented anterior to the top and dorsal to the right. Photos a and b are in bright field optics; c, d and f in phase contrast; and e in Nomarski optics. a, Class I embryo of stmA1; note the continuous ventral and dorsal cuticles and normal head skeletal structures (hs) and anal structures (as); the region of the three-hour-old embryo that gives rise to the dorsal and ventral neuroectoderm is marked by a dotted line. b, Class II embryo of stmA2 showing large dorsal cuticle hole, reduced head skeleton and an overall reduction in the cuticle area. c and d, Class III embryos of stmA1 and stmA2 showing large cuticle holes in the ventral and dorsal areas. e, Class IV embryo of stmA2 showing a narrow strip of dorsal cuticle. f, Class V embryo showing no continuous cuticle. 90 Journal of Genetics, Vol. 80, No. 2, August 2001 Mutations at stambh A in Drosophila melanogaster Table 8. Distribution of heat-killed embryos into cuticle loss classes I to V. % Embryos* in cuticle loss class Age of eggs (hAEL) Temperature (ºC) 0 33 3 33 Genotype I II III IV V + stmA1 stmA2 + stmA1 stmA2 79 34 23 89 44 22 21 37 61 11 16 2 0 12 2 0 30 62 0 8 4 0 2 4 0 1 0 0 8 10 hAEL, Hours after egg laying. *Rounded off to the nearest integer. For description of classes see Materials and methods. in some way involved in the process that leads to cuticle loss. We conclude that stmA is a maternally active gene that is needed for normal neural and ectodermal development in the embryo. stmA+ is necessary for female germline viability Figure 5. Frequency of heat-induced cuticleless embryos in stmA and wild-type embryos. with severe cuticle loss (classes II–V) was three-fold to seven-fold lower than in stmA embryos. We conclude from this experiment that heat-induced embryonic lethality is associated with cuticle loss both in stmA and in wild-type Canton-S genotypes, its severity being much greater among stmA genotypes. These observations demonstrate that the wild-type function of stmA is needed postzygotically during the first three hours of embryogenesis to ensure proper neuroectodermal development. Heat-induced cuticle loss is maternally influenced It has been reported that several neurogenic genes such as Notch, mastermind and Enhancer of split exert a maternal influence over cell-fate choice in the neuroectoderm (Lehmann et al. 1983; Schrons et al. 1992). To see if stmA has a similar maternal effect, reciprocal crosses were made between stmA1, stmA2 and Canton-S, and the stmA / + embryos heat-shocked. The results showed that in general the frequency of embryonic lethality and cuticle loss (table 9) was far greater when the stmA / + embryos were derived from stmA mothers than when derived from wild-type mothers. stmA mothers obviously produce a heat-sensitive product that persists in the cytoplasm and is Maternal germline clones of stmA7 and stmA12 were induced using mitotic recombination in the ovoD background. The purpose of inducing maternal germline clones of stmA embryonic lethal alleles was to ask if (i) homozygous stmAl / stmAl clones survived and (ii) if the clones on survival exerted any maternal influence over postzygotic development. P[ovoD] is inserted at 55DE while stmA is located proximally at 44D1.2 (figure 6a). In this given situation two types of mitotic recombination events are expected. The first category of recombination events is expected in region I (see figure 6b) and is expected to yield stmAl / stmAl clones. The other category of recombination events is expected in region II between stmA and P[ovoD] and would yield stmAl / + clones (figure 6c). We have not used any closely linked flanking marker between stmA and P[ovoD] to distinguish event I from event II. We have instead resorted to simple but robust genetic testing to determine the genotype of the F1 progeny of clone-bearing females. Clone-bearing females were crossed to wild-type males and all the F1 progeny were individually test-crossed to b stmA1 flies. Each F2 test cross family was scored for segregation for b stmA1 / + vs b stmA1 / stmAl flies. The F2 phenotypic ratios determined the F1 parental genotype and thereby the genotype of the maternal germline. The null hypothesis was simply that if the germline genotype was stmAl / stmAl all the F1 flies would be of the stmAl / + genotype and each F2 family would segregate for b stmA1 / + vs b stmA1 / stmAl in a 1 : 1 ratio. Clones in the stmA + / + P[ovoD] controls were induced at a frequency of 6.72% (table 10). These clones represent all the mitotic recombination events between the centromere and stmA (region I) and between stmA and P[ovoD] (region II). Clone frequencies in Journal of Genetics, Vol. 80, No. 2, August 2001 91 M. Kumar et al. stmA7 + / + P[ovoD] and stmA12+ / + P[ovoD] were 2.3% and 2.8% respectively, which are roughly half that of the control. These low frequencies were observed despite testing twice the number of females of stmA7 + / + P[ovoD] and stmA12+ / + P[ovoD]. F2 progeny testing of the adults derived from the clone-bearing females (mated to wild-type males) showed that the clones induced in the 13 clone-bearing females (eight from stmA12 + / + P[ovoD] and five from stmA7 + / +P[ovoD]) were all of stmA / + genotype and none were homozygous stmA / stmA. Data on testing of the eight germline clonebearing females derived from stmA12 + / + P[ovoD] are Table 9. Heat-induced lethality and cuticle loss among embryos derived from reciprocal matings involving stmA1, stmA2 and Canton-S . 0-hour-old eggs 3-hour-old eggs Parents (female × male) % EL (N) % CLE % EL (N) % CLE + × A1 A1 × + A1 × A1 + × A2 A2 × + A2 × A2 +×+ 22.0 (500) 91.8 (500) 89.7 (475) 41.7 (525) 48.2 (541) 54.4 (428) 29.3 (1019) 22 72 76 20 63 85 25 14.5 (359) 56.7 (466) 99.2 (495) 23.5 (375) 78.1 (375) 92.7 (301) 29.1 (760) 21 68 70 11 82 80 12 % EL, Per cent embryonic lethality; N, number of embryos studied; % CLE, per cent cuticleless embryos. Clone frequency Chromosome combination Clone genotype stmA7 stmA12 1,3 2,3 1,4 2,4 stmA+ / stmA+ + ovoD / stmA+ stmA+ / + ovoD + ovoD / + ovoD 0 0 0 0 0 0 0 0 Chromosome combination 1,3 2,3 1,4 2,4 Clone frequency Clone genotype stmA7 stmA12 stmA+ / ++ stmA ovoD / ++ stmA+ / + ovoD 2.3 0 0 0 2.81 0 0 0 stmA ovoD / + ovoD Figure 6. a, Relative positions of stmA and P[ovoD] with respect to the centromere in mitotic chromosomes. b and c, Consequences of mitotic recombination in region I (b) and in region II (c) with the frequencies of expected categories of clone from each of the recombination events. 92 Journal of Genetics, Vol. 80, No. 2, August 2001 Mutations at stambh A in Drosophila melanogaster presented in table 11 to illustrate the nature of the tests that were done. Eggs derived from mating the clone-bearing females with wild-type males also showed control levels of embryonic lethality (ranging from 3% to 6%) and did not show any consistent pattern of cuticle loss as may have been expected if the clones were of the stmA genotype. In conclusion, failure to recover stmA / stmA clones indicates that stmA7 and stmA12 are lethal to the female germline. Discussion This study was aimed at understanding the genetics of the stmA locus and determining its role in fly development. Twelve stmA alleles isolated in four different screens are reported. The alleles fall into two phenotypic categories of reversible ts paralytic homozygous viable (four alleles) and unconditional lethals (eight alleles). The 12 alleles fall into two mutually complementing groups, groups II and III, and a third group I that does not complement members of either group II or group III. Groups I and II consist of unconditional lethals while group III includes ts paralytic as well as unconditional lethals. No ts paralytic allele belonging to group I has so far been isolated. This might imply that stmA alleles with unconditional lethality are mutations causing very severe impairment of gene activity or total loss of gene function while ts paralysis is Table 10. Genotype due to a less drastic change. The paralytic phenotypes of various heteroallelic combinations (table 5) do not however follow this simple assumption that lethality is caused by more severe mutations than ts paralysis. If the assumption were correct, paralytic (viable) / lethal heterozygotes ought to have had stronger paralytic phenotypes than their respective paralytic (viable) homozygotes. Eleven of 32 trans heterozygotes showed a weaker paralytic phenotype than their respective homozygotes. Interestingly stmAP1, which is recessive to the wild type, shows weaker paralysis when heterozygous over most lethal alleles. Absence of a simple correlation between the phenotypes of the various trans heterozygotes and their homozygotes supports the view that the various mutations do not fall into a single functional group. Intragenic complementation among stmA alleles also points towards a complex genetic nature of the locus. Intragenic complementation is typically seen in genes that have more than one functional domain and whose protein products are multimeric in nature (Gepner et al. 1996; Grant et al. 1998). It has been demonstrated that lethal alleles of the gene shibire, which also gives rise to ts paralytic mutations, show intragenic complementation (Grant et al. 1998). shibire encodes the Drosophila homologue of dynamin (a GTPase), which is known to have multiple functional domains (Grant et al. 1998). Molecular analysis of stmA will provide answers to similar questions about its structure and function. Frequency of female germline clones of stmA. Age (hAEL) at irradiation % Clone bearing females (N) Number of females tested 60 ± 12 84 ± 12 108 ± 12 108 ± 12 108 ± 12 4.89 (15) 6.16 (17) 6.72 (8) 2.81 (8) 2.30 (5) 307 276 119 285 215 + / P[ovoD] stmA12 + / + P[ovoD] stmA7 + / + P[ovoD] hAEL, Age at time of irradiation in hours after egg laying; N, number of clone-bearing females. Table 11. Analysis of embryos and adult progeny of the eight germline clone-bearing females derived from irradiating stmA12 + / + P[ovoD] larvae. The germline clone-bearing females were mated to wild-type males. Unhatched eggs (F1) Female Eggs laid N (%) unfertilized N (%) fertilized Fertilized & hatched eggs (F1) 1 2 3 4 5 6 7 8 32 26 80 10 31 235 66 31 6 (18.7) 6 (23.0) 17 (17.5) 1 (10.0) 4 (12.9) 8 (3.4) 10 (15.1) 4 (12.9) 0 0 3 (3.15) 0 0 10 (4.26) 0 0 26 20 63 9 27 217 56 27 Adults recovered (F1) 26 18 52 8 26 198 54 25 Journal of Genetics, Vol. 80, No. 2, August 2001 F1 genotypes F2 test cross determined from F2 families tested +/+ for segregation + stmA12 / + 15 15 33 7 20 103 35 20 4 9 12 2 12 42 15 3 11 6 21 5 8 61 20 17 93 M. Kumar et al. Towards this objective, stmA has been precisely located to the polytene map position 44D1.2, and P inserts using wild-type P elements from a Birm-2 chromosomal source have been generated. Birm-2 P elements do not have any selectable markers or any plasmid rescue sequences that simplify cloning. Attempts are in progress to generate rescuable PlacW inserts in stmA by two methods: (i) by P element exchange involving the Birm-2 and an X-linked PlacW element (Sepp and Auld 1999) and (ii) by locally mobilizing a PlacW from position 44D3.4 into stmA. Role in development Genetic analysis of behavioural defects through the study of mutants of single genes in Drosophila and several other animals has provided valuable insight into the regulation of neural functions and development (Heisenberg 1997). Several behavioural mutants are associated with abnormal neural development (Hall 1982; Poodry 1990). It was earlier suggested (Chandrashekaran and Sarla 1993) that stmA was involved in neuroectodermal development on the basis of observations that embryos of two unconditional lethal alleles showed neural hypertrophy and cuticle hypotrophy. The present study has revealed that embryos of the ts paralytic allele stmA1 show mild neural hypertrophy even at the permissive temperature of 23ºC. This neural abnormality at 23ºC is consistent with the observation that stmA1 adults show abnormal behaviour at 23ºC. No abnormalities in neural connections or cuticle loss were observed in stmA1 embryos at this temperature. After a short heat shock at temperatures between 31°C and 35ºC, however, stmA1 and stmA2 embryos develop a strong neurogenic phenotype showing strong to extreme cuticle loss. It has also been found that stmA is maternally active and is needed during early embryogenesis between zero and three hours for proper ectodermal differentiation.. Coinduction of ectodermal hypotrophy and neuronal hypertrophy by heat treatment has not yet been demonstrated experimentally. Nevertheless there are reasons to expect neuronal hypertrophy in heat-killed embryos because embryos with zygotic unconditional lethal alleles of stmA show strong neuronal hypertrophy and those with conditional ts paralytic alleles display mild hypertrophy. It is interesting that a similar heat-induced neurogenic phenotype is seen in the ts paralytic mutation shibire (Poodry 1990). shibire encodes the Drosophila homologue of dynamin and is needed for recycling of synaptic vesicles, and is unrelated structurally and functionally to the Notch-Delta group of neurogenic genes. This study also shows that stmA is needed for the viability of the maternal germline. The functions of the neurogenic genes Notch and Delta are also required for the viability of the female germline and participate in cell–cell communication during oogenesis (Ruohola et al. 1991). 94 The term ‘neurogenic’ with reference to stmA is used merely to refer to the phenotype of neural hypertrophy and cuticle hypotrophy. In Drosophila literature the term ‘neurogenic’ is strongly linked with the first few ‘neurogenic’ genes of the Notch-Delta category, whose mutations produce such a phenotype (Lehmann et al. 1983). The Notch-Delta neurogenic genes control the choice of cell fate between neuroderm and ectoderm in the early stages of Drosophila embryonic development (among a host of other cell-fate choice decisions) by influencing cell–cell communication. Several of them encode epidermal-growth-factor-like proteins, transmembrane receptors and transcription factors (Campos-Ortega 1993). Using the term ‘neurogenic’ with reference to stmA merely implies that the process of neural vs epidermal fate might involve the function of stmA. It remains to be demonstrated that stmA is developmentally related to the ‘neurogenic’ genes. It has been shown previously (Chandrashekaran 1993) that stmA adults resist the lethal effects of the sodiumchannel-binding neurotoxin veratridine at 23–24ºC. Such resistance may be attributed to altered binding of veratridine to voltage-gated sodium channel subunits due to a constitutive defect in membrane-related properties of stmA mutant flies. The observation that stmA flies show behavioural defects and a mild neural hypertrophy at 23– 24ºC supports the idea of a constitutive neuronal defect. It is evident that constitutive defect in the ts paralytic alleles gets exaggerated at higher temperatures leading to cuticle loss and death in embryos and paralysis in adults. In conclusion, the results presented demonstrate that stmA is a functionally complex locus. It is functional both maternally and zygotically and is needed for normal neuronal development in the early embryo, in normal behavioural functions in the adult, and for the viability of the maternal germline. Essential information to facilitate cloning and molecular analysis have been obtained by way of precise physical location and transposon tagging of the gene. Acknowledgements I take this opportunity to thank Prof. Balram Sharma of the Division of Genetics for critically reading this manuscript in its final stages of preparation. We thank Dr K. S. Krishnan for deficiency stocks, Pradip Sinha for the P[ovoD] stock, and Dr Veronica Rodrigues for gifting us Mab 22C10 and letting us use her laboratory. References Benzer S. 1967 Behavioral mutants of Drosophila isolated by counter current distribution. Proc. Natl. Acad. Sci. USA 58, 1112–1119. Campos-Ortega J. A. 1993 Early neurogenesis in Drosophila melanogaster. In The development of Drosophila melanogaster (ed. M. Bate and A. Martinez-Arias), pp. 1091–1130. Cold Spring Harbor Laboratory Press, Cold Spring Harbor. Journal of Genetics, Vol. 80, No. 2, August 2001 Mutations at stambh A in Drosophila melanogaster Catterall W. A. 1986 Properties of voltage-sensitive sodium channels. Annu. Rev. Biochem. 55, 953–986. Chandrashekaran S. 1993 Mutations at the stmA locus of Drosophila melanogaster confer resistance to the sodium channel neuropoison veratridine. Curr. Sci. 65, 80–82. Chandrashekaran S. and Sarla N. 1993 Phenotypes of lethal alleles of ts paralytic mutant stmA of Drosophila melanogaster suggest its neurogenic function. Genetica 90, 61–71. Chou T. B., Noll E. and Perrimon N. 1993 Autosomal P (ovoD1) dominant female sterile insertions in Drosophila and their use in generating germ line chimaeras. Development 119, 1359– 1369. Gepner J., Li M., Ludmann S., Kortas C. and Boylan K. 1996 Cytoplasmic dynein function is essential in Drosophila melanogaster. Genetics 142, 865–878. Grant D., Unadkat S., Katzen A., Krishnan K. S. and Ramaswami M. 1998 Probable mechanisms underlying interallelic complementation and temperature sensitivity of mutations at the shi locus of Drosophila melanogaster. Genetics 149, 1019–1030. Hall J. C. 1982 Genetics of early neurogenesis in Drosophila. Quart. Rev. Biophys. 15, 223–479. Heisenberg M. 1997 Genetic approach to neuroethology. BioEssays 19, 1065–1073. Kumar M. 1993 Genetic analysis of the 42A polytene chromosome section of Drosophila melanogaster. Ph.D. thesis, Indian Agricultural Research Institute, New Delhi, India. Lehmann R., Dietrich U., Jiminez F. and Campos-Ortega J. A.. 1983 On the phenotype and development of mutants of early neurogenesis in Drosophila melanogaster. Roux’s Arch. Dev. Biol. 192, 62–74. Loughney K., Kreber R. and Ganetzky B. 1989 Molecular analysis of the para locus, a sodium channel gene in Drosophila. Cell 58, 1143–1154. Poodry C. A. 1990 shibire, a neurogenic mutant of Drosophila. Dev. Biol. 138, 464–472. Robertson H. M., Preston C. R., Phillis R. W., Johnson-Sclitz D. M., Benz W. K. and Engels W. R. 1988 A stable genomic source of P element transposase in Drosophila melanogaster. Genetics. 118, 461–470. Ruohola H., Bremer K. A., Baker D., Swedlow J. R., Jan L. Y. and Jan Y. N. 1991 Role of neurogenic genes in establishment of follicle cell fate and oocyte polarity during oogenesis in Drosophila. Cell 66, 433–449. Schrons H., Knust E. and Campos-Ortega J. A. 1992 The Enhancer of split complex and adjacent genes in the 96F region of Drosophila melanogaster are required for the segregation of neural and epidermal progenitor cells. Genetics 132, 481–503. Sepp K. J. and Auld V. J. 1999 Conversion of lacZ enhancer trap lines to Gal4 lines using targeted transposition in Drosophila. Genetics 151, 1093–1101. Shyngle J. and Sharma R. P. 1985 Studies on paralysis and development of second chromosome ts paralytic mutants of Drosophila melanogaster. Indian J. Exp. Biol. 23, 235–240. van der Meer J. 1977 Optical clean and permanent whole mount preparation for phase contrast microscopy of cuticular structure of insect larvae. Dros. Inf. Serv. 52, 160. Received 23 May 2001; in revised form 31 August 2001 Journal of Genetics, Vol. 80, No. 2, August 2001 95