* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Gpr126 is essential for peripheral nerve development and
Feature detection (nervous system) wikipedia , lookup
Subventricular zone wikipedia , lookup
Neuroanatomy wikipedia , lookup
Microneurography wikipedia , lookup
Development of the nervous system wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Axon guidance wikipedia , lookup
Synaptogenesis wikipedia , lookup
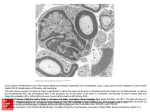
RESEARCH ARTICLE 2673 Development 138, 2673-2680 (2011) doi:10.1242/dev.062224 © 2011. Published by The Company of Biologists Ltd Gpr126 is essential for peripheral nerve development and myelination in mammals Kelly R. Monk1,*, Kazuo Oshima2, Simone Jörs2, Stefan Heller2 and William S. Talbot1,† SUMMARY In peripheral nerves, Schwann cells form the myelin sheath that insulates axons and allows rapid propagation of action potentials. Although a number of regulators of Schwann cell development are known, the signaling pathways that control myelination are incompletely understood. In this study, we show that Gpr126 is essential for myelination and other aspects of peripheral nerve development in mammals. A mutation in Gpr126 causes a severe congenital hypomyelinating peripheral neuropathy in mice, and expression of differentiated Schwann cell markers, including Pou3f1, Egr2, myelin protein zero and myelin basic protein, is reduced. Ultrastructural studies of Gpr126–/– mice showed that axonal sorting by Schwann cells is delayed, Remak bundles (non-myelinating Schwann cells associated with small caliber axons) are not observed, and Schwann cells are ultimately arrested at the promyelinating stage. Additionally, ectopic perineurial fibroblasts form aberrant fascicles throughout the endoneurium of the mutant sciatic nerve. This analysis shows that Gpr126 is required for Schwann cell myelination in mammals, and defines new roles for Gpr126 in axonal sorting, formation of mature non-myelinating Schwann cells and organization of the perineurium. INTRODUCTION Myelin is a multilayered membrane formed by glial cells around axons in the vertebrate nervous system. In the peripheral nervous system (PNS), Schwann cells form the myelin sheath by wrapping their membrane around an axonal segment many times. Schwann cell precursors arise from neural crest progenitor cells, and previous work has defined several steps in the developmental progression leading to mature Schwann cells (Jessen and Mirsky, 2005). During embryogenesis, Schwann cell precursors co-migrate with axons in the developing PNS and differentiate into immature Schwann cells that remain associated with many axons. Immature Schwann cells may become either non-myelinating or myelinating Schwann cells. Non-myelinating Schwann cells remain associated with multiple small-caliber axons. These axons are ensheathed by non-myelinating Schwann cells in Remak bundles, but are not myelinated. Other immature Schwann cells begin to sort largecaliber axons and develop into promyelinating Schwann cells that associate with a single axonal segment. Promyelinating Schwann cells differentiate into myelinating Schwann cells, which repeatedly wrap their membrane around an axonal segment to eventually form a compact myelin sheath. Investigations in mammals have defined a number of essential regulators of Schwann cell development, including the axonal signal neuregulin 1 and the Schwann cell transcription factors Pou3f1 (also known as Oct-6), Pou3f2 (also known as Brn-2) and Egr2 (also known as Krox-20) (Nave and Salzer, 2006; Svaren and Meijer, 2008). Neuregulin 1 (Nrg1) signaling through ErbB receptors is crucial for many steps in Schwann cell development, including 1 Department of Developmental Biology, Stanford University School of Medicine, Stanford, CA 94305, USA. 2Department of Otolaryngology-Head and Neck Surgery, Stanford University School of Medicine, Stanford, CA 94305, USA. *Present address: Department of Developmental Biology, Washington University School of Medicine, St Louis, MO 63110, USA † Author for correspondence ([email protected]) Accepted 6 April 2011 proliferation, ensheathment and myelination (Garratt et al., 2000; Michailov et al., 2004; Taveggia et al., 2005). The transcription factors Pou3f1, Pou3f2 and Egr2 are required for the development of mature myelinating Schwann cells. Pou3f1 and Pou3f2 are required for the timely differentiation of myelinating Schwann cells; Schwann cells in Pou3f1-null mice transiently arrest at the promyelinating stage and deletion of both Pou3f1 and Pou3f2 exacerbates this phenotype (Bermingham et al., 1996; Jaegle et al., 1996; Jaegle et al., 2003). Schwann cells in Egr2-null mice remain arrested at the promyelinating stage and fail to wrap an axonal segment more than 1.5 turns (Topilko et al., 1994; Zorick et al., 1999). Less is known about the genes controlling the development of non-myelinating Schwann cells, but these cells express many of the same markers as immature Schwann cells (Jessen and Mirsky, 2002). We recently reported that the orphan G-protein-coupled receptor Gpr126 is required autonomously in Schwann cells for the expression of pou3f1 and egr2, and that Gpr126 is essential for promyelinating Schwann cells to initiate myelination in zebrafish (Monk et al., 2009). In the present study, we show that this function of Gpr126 is conserved in mammals through the analysis of Gpr126 knockout mice. As in zebrafish, Schwann cells in Gpr126–/– mutant mice are arrested at the promyelinating stage. Mutant mice have limb posture abnormalities, are smaller than littermates and die before weaning. In mutant sciatic nerves, we also observe delayed sorting of axons by Schwann cells, aberrant fasciculation throughout the endoneurium by perineurial fibroblasts, progressive loss of axons in the postnatal period and an absence of Remak bundles. Our findings show that Gpr126 is essential for several aspects of peripheral nerve development in mammals. MATERIALS AND METHODS Mice Gpr126 knockout mice were generated by Lexicon Pharmaceuticals (The Woodlands, TX, USA) and heterozygous animals were obtained from Taconic (Hudson, NY, USA; catalog number TF0909) on a mixed background (129/SvEv-C57BL/6). These mice and their offspring were crossed to generate the animals analyzed in this study. We used the following PCR primers to determine the genotype of the mutant mice: DEVELOPMENT KEY WORDS: Schwann cell, Myelin, Gpr126, Mouse 2674 RESEARCH ARTICLE RT-PCR and quantitative real-time PCR For standard RT-PCR, we isolated RNA from Gpr126–/– and heterozygote sibling sciatic nerves at postnatal day (P)4 using the RNeasy Micro Kit (Qiagen) according to the manufacturer’s instructions. We reverse transcribed the RNA using the SuperScript First-Strand synthesis system for RT-PCR (Invitrogen) with Superscript II reverse transcriptase and random hexamers per manufacturer’s instructions. Samples lacking reverse transcriptase were used to control for genomic DNA contamination. We amplified Gapdh as a control for each sample using published primers (Peirano et al., 2000). Sox10, Egr2 and Dhh were amplified as described [Sox10 and Egr2 (Peirano et al., 2000); Dhh (Parmantier et al., 1999)]. We used the following primers to amplify Gpr126: 5⬘-CCAAAGTTGGCAATGAAGGT-3⬘ and 5⬘-GCTGGATCAGGTAGGAACCA-3⬘. For quantitative real-time PCR (qPCR), the same heterozygote P4 sciatic nerve cDNA and primers were used as described above. Mouse dorsal root ganglia (DRGs) were collected from embryonic day (E)13.5 and neurons were purified as described (Sasaki et al., 2009). RNA was extracted from DRG neurons using Ribozol (Amresco) and cDNA was synthesized using QScript cDNA Supermix (VWR) according to the manufacturer’s instructions. qPCR was performed in an ABI 7500 using SYBR Green Master Mix (ABI) according to standard protocols. Transmission electron microscopy Sciatic nerves and optic nerves were removed from mice of the indicated ages and genotypes and immediately immersed in fresh fixative (4% paraformaldehyde, 2% glutaraldehyde in 0.1M sodium cacodylate, pH 7.4) for three hours. Nerves were contrasted by postfixation in 2% osmium tetraoxide in 0.1M sodium cacodylate, pH 7.4, dehydrated, and embedded in epoxy resin (EMBed-812, Electron Microscopy Sciences). We stained semithin sections (1 m) with Toluidine Blue for light microscope examination (Zeiss Axioplan2). Ultrathin sections (70 nm) were stained and imaged as described (Pogoda et al., 2006). We examined six siblings [three wild type (WT), three Gpr126+/–] and three mutants at P1; five siblings (two WT, three Gpr126+/–) and three mutants at P4; five siblings (two WT, three Gpr126+/–) and three mutants at P8; five siblings (one WT, four Gpr126+/–) and four mutants at P12. Morphometric analysis For P1 animals (n2 pairs of WT and Gpr126–/– siblings), images of the sciatic nerve were taken in an overlapping series and montaged in Photoshop. We then counted the total number of axons in P1 sciatic nerve. For WT animals, the tibial branch of the nerve was analyzed; Gpr126 mutant nerves were difficult to dissect and we did not observe branches in the nerve in the regions examined. For P8 WT and Gpr126+/– siblings (n3: one WT and two Gpr126+/–), we imaged Toluidine Blue-stained semi-thin sections (see above) on a Zeiss Axioplan2 and digitally imaged the entire nerve with a Zeiss AxioCam. We then counted the total number of myelinated axons in the tibial branch of P8 sciatic nerve. To estimate the total number of axons in WT nerve, we imaged ten randomly selected regions of the same nerves using ultrathin sections at 8,000⫻ magnification. We then counted the total number of unmyelinated axons and myelinated axons in these regions and obtained an average ratio of unmyelinated:myelinated axons (576:168). We used this ratio and the counted number of myelinated axons to estimate the total number of axons in WT sciatic nerve. For Gpr126–/– mutants (n3), we imaged ultrathin sections (see above) at 4000⫻ magnification. Images of the entire nerve were taken in an overlapping series and montaged in Photoshop. We then counted the total number of axons in P8 sciatic nerve. Immunohistochemistry Cochlear dissections, sample preparation, cryosectioning and immunostaining were performed as described (Oshima et al., 2009). We used the following primary antibodies: rat-anti-myelin basic protein (1:10, AbD Serotec), rabbit-anti-peripherin (1:100, Millipore Bioscience Research Reagents), goat-anti-Pou3f1 (1:100, Santa Cruz Biotechnology), mouseanti-myelin protein zero (1:1000) (Vargas et al., 2010) and rabbit-antineurofilament-M (1:200, Chemicon). Samples were incubated with appropriate fluorescently labeled secondary antibodies (1:1000, Molecular Probes; 1:200, Jackson ImmunoResearch), visualized by epi-fluorescence microscopy (Zeiss Axioplan2 for sciatic nerves and Zeiss Axioimager for cochleae) and digitally photographed (Zeiss AxioCam). We examined three Gpr126+/– littermates and two Gpr126–/– mutants at P8 for sciatic nerve stains, and we examined five littermates (two wild type, three Gpr126+/–) and three Gpr126–/– mutants at P14 for cochlear stains. Statistical analysis For axon quantification, statistical comparisons were made using a twotailed t-test. Differences were considered to be significant at P<0.05. RESULTS Nerve and limb defects in Gpr126–/– mice Starting with a genetic screen for mutants with abnormal development of myelinated axons, we previously described an essential role for Gpr126 in Schwann cell myelination in zebrafish (Monk et al., 2009). To determine whether the function of Gpr126 is conserved in mammalian myelination, we obtained and analyzed Gpr126 knockout mice from Taconic/Lexicon. In the mutant allele, exons 2 and 3 are replaced by a selection cassette (Fig. 1A,B). By RT-PCR using primers that span exons 3 and 4, we detected Gpr126 mRNA in Gpr126+/– sciatic nerves, but not in wild-type neurons purified from E13.5 dorsal root ganglia (DRG; Fig. 1C,D). Because most mRNAs in the sciatic nerve are transcribed by Schwann cells, this result suggests that, as in zebrafish, mammalian Schwann cells express Gpr126. As expected for a deletion allele lacking exons 2 and 3, mutant sciatic nerves did not express detectable Gpr126 mRNA using primers that span exons 3 and 4 (Fig. 1C). Crosses of Gpr126+/– heterozygotes yielded <25% mutant pups at birth (26 homozygous mutants out of 334 pups; 7.8%), indicating that the majority of Gpr126 mutants did not survive to birth. At birth, mutant pups were generally the same size as their wild type (WT) and heterozygous siblings, but they failed to grow at equal rates and were substantially smaller than littermates within a week of birth (Fig. 1E; see Movie 1 in the supplementary material). Gpr126–/– mice died before weaning, usually between postnatal day (P)8 and P16. Gpr126–/– mice that survived to P12 were generally immotile; when movement was occasionally attempted, the mutants flailed their limbs and locomotion was uncoordinated (see Movie 1 in the supplementary material). Mutants were distinguishable from their WT and heterozygous littermates by abnormal joint contractures of the forelimbs and hindlimbs (Fig. 1E; see Movie 1 in the supplementary material). This phenotype was sometimes observed as early as birth, and was readily observable by P4. This limb phenotype is reminiscent of human patients with arthrogryposis multiplex congenita (AMC) (Bamshad et al., 2009) and has also been reported in mouse Adam22 mutants (Nishino et al., 2010) and mouse claw paw mutants (Henry et al., 1991; Darbas et al., 2004), which have a mutation in the Lgi4 gene (Bermingham et al., 2006). In addition to these limb posture abnormalities, sciatic nerves in Gpr126–/– mice were severely reduced in size with a hypomyelinated (i.e. translucent) appearance (Fig. 1F,G). To learn more about the defects in Gpr126–/– sciatic nerves, we examined several markers of Schwann cell development by RTPCR using cDNA template prepared from P4 sciatic nerve. Gpr126–/– sciatic nerves expressed Sox10 (Fig. 1C), a transcription factor crucial for early Schwann cell development (Kuhlbrodt et al., 1998; Britsch et al., 2001). This result suggests that Schwann cells DEVELOPMENT Primer A, 5⬘-GTAGGATTGTTGCTTTATTATAC-3⬘; Primer B, 5⬘CCTGATCCTGACCATTCGC-3⬘; and Primer C, 5⬘-CCCTAGGAATGCTCGTCAAGA-3⬘. All animal procedures were approved by Stanford University’s Administrative Panel on Laboratory Animal Care or by Washington University’s Institutional Animal Care and Use Committee. Development 138 (13) Gpr126 is essential for PNS myelination RESEARCH ARTICLE 2675 are present and expressing Sox10 in the mutant nerves, similar to our previous studies in zebrafish (Monk et al., 2009). Expression of other Schwann cell markers, including Dhh and Egr2, was not detectable in Gpr126–/– nerve (Fig. 1C). Additionally, Pou3f1 and myelin basic protein (Mbp) expression were not detected in Gpr126–/– sciatic nerve (Fig. 2B,D). This analysis provides evidence that Schwann cells are present in Gpr126–/– peripheral nerve, but that these cells do not develop normally. Multiple ultrastructural abnormalities are observed in Gpr126–/– nerves To determine whether Gpr126 is required for the differentiation of myelinating Schwann cells in mammals, we examined myelination and nerve ultrastructure at several developmental time points in WT, Gpr126+/– and Gpr126–/– mice (see Materials and methods). We did not observe any abnormalities in heterozygous animals, and the development of myelinated axons was similar in WT and Gpr126+/– sciatic nerves (see Fig. S1 in the supplementary material; Fig. 3). Additionally, myelination of optic nerve axons by oligodendrocytes, the myelinating glial cells of the central nervous system (CNS), appeared normal in Gpr126–/– mutants at P12 (n4; see Fig. S2 in the supplementary material), suggesting that Gpr126 is not essential for myelination in the CNS. The ultrastructural studies revealed striking abnormalities in Gpr126–/– peripheral nerve at P1, the earliest time point examined. In P1 WT and Gpr126+/– animals, axonal sorting was well underway in sciatic nerve; many Schwann cells had reached the promyelinating stage, and a few thinly myelinated axons were observed (Fig. 3A). By contrast, Schwann cell development was markedly delayed in Gpr126–/– nerves. In the mutant sciatic nerve at P1, most Schwann cells were at early sorting stages located at the periphery of axon bundles and had sorted very few axons away from the bundles (Fig. 3E). From P4 to P12 in WT and Gpr126+/– animals, large caliber axons were progressively myelinated, and Remak bundles developed as non-myelinating Schwann cells ensheathed small caliber axons (Fig. 3B-D; Fig. 4A). From P4 to P12 in Gpr126–/– nerves, Schwann cells eventually sorted axons and formed a basal lamina, but all Schwann cells that were associated with axons in a 1:1 relationship were arrested at the promyelinating stage and myelin was never observed (Fig. 3F-H; Fig. 4B,E). Additionally, we did not observe developing Remak bundles in Gpr126–/– sciatic nerve (Fig. 3G,H; Fig. 4B). To determine whether the loss of Remak bundles might reflect the loss of small caliber axons that are normally ensheathed by nonmyelinating Schwann cells, we analyzed the axons in Gpr126–/– sciatic nerves. At P1, the total number of axons in Gpr126–/– sciatic nerve (5853±2109 s.d.) was somewhat reduced compared with the total number of axons in WT nerve (9808±2720 s.d.; P0.07), although the difference in axon number was not significant. The reduction in axon number was far more pronounced at later stages. At P8, the total number of axons in Gpr126–/– sciatic nerve (1314±383 s.d.) was somewhat less than the number of myelinated DEVELOPMENT Fig. 1. Gpr126–/– mice have limb and nerve abnormalities. (A)Diagrams of Gpr126 gene (top) and Gpr126 protein (bottom). In the Gpr126 gene, exons 1 and 23 are denoted, as is the targeted locus (red arrows). The protein diagram depicts functional domains in Gpr126: CUB domain (Complement, Uegf, Bmp1; CUB), a Pentraxin domain (PTX), a G protein-coupled receptor proteolytic site (GPS) and a type II seven-transmembrane domain (7TM) (Stehlik et al., 2004). (B)Diagram depicting the targeting strategy employed to generate Gpr126–/– mice. (C)RT-PCR analysis of expression of Schwann cell developmental markers in cDNA derived from P4 Gpr126+/– (+/–) or Gpr126–/– (–/–) sciatic nerve. Sox10 is expressed in both +/– and –/– nerve, but expression of Gpr126, Dhh and Egr2 is not detectable in mutant nerve. Gapdh is included as a loading control, and samples lacking reverse transcriptase (RT–) in the cDNA reaction mixture show no amplification. (D)Table showing average cycle threshold of expression for Gapdh and Gpr126 in neurons purified from E12.5 dorsal root ganglia (DRG) and in P4 sciatic nerve (sciatic), as determined by quantitative real-time PCR (qPCR). We did not detect Gpr126 in DRG neurons by qPCR, although with extended cycling under conditions of high sensitivity using traditional RT-PCR, weak Gpr126 expression was observed in the same DRG neuron sample (data not shown). (E)Wild-type (WT) and Gpr126–/– littermates at P8. Gpr126–/– mutants are smaller than their siblings and display abnormal joint contractures in the forelimbs and hindlimbs (arrowheads). (F,G)Sciatic nerves from P12 littermates (arrows). (F)WT sibling nerves are white and opaque; a Gpr126+/– (+/–) animal is shown. (G)By contrast, Gpr126–/– nerves are thinner than wild-type nerves and are translucent, indicative of hypomyelination. Fig. 2. Gpr126–/– peripheral nerve lacks late Schwann cell markers. (A)Pou3f1 is expressed in nuclei of Gpr126+/– nerve at P8 (A⬘,A⬙, arrowheads). (B)Pou3f1 is not detected in nuclei of Gpr126–/– nerve at P8 (B⬘,B⬙). (C)Myelin basic protein (Mbp; C,C⬙,C) and Peripherin (C⬘) are expressed in Gpr126+/– sciatic nerve at P8. (D)Mbp is not detected in Gpr126–/– sciatic nerve at P8 (D,D⬙,D). Peripherin is expressed in Gpr126–/– sciatic nerve at P8 (D⬙). Boxed areas in C⬙ and D⬙ are enlarged in C and D, respectively. axons in WT nerve (1855±275 s.d.), and much less than the estimated number of total axons in WT nerve (extrapolated value8216±1220, P0.006). To determine whether small caliber Cfiber axons were absent in the mutants, we examined the expression of peripherin, a marker of C-fibers (Goldstein et al., 1991). Gpr126–/– sciatic nerves expressed peripherin (Fig. 2D), indicating that the loss of Remak bundles is not simply due to absence of small Development 138 (13) caliber C-fiber axons. This analysis shows that Gpr126 is required for the development of myelinating Schwann cells in mammals and suggests that Gpr126 also has a role in the development of nonmyelinating Schwann cells. In addition, axon number was markedly reduced in Gpr126–/– nerves at P8, indicating that Gpr126 function is required for the maintenance of axons. A fasciculation defect was evident in Gpr126–/– nerves as early as P4. Throughout the endoneurium in mutant nerves, flattened cells encircled groups of axons and Schwann cells, forming minifascicles (see Fig. S1 in the supplementary material; Fig. 3F,G; Fig. 4B). These flattened cells are most likely to be perineurial fibroblasts, as they contained numerous caveolae and possessed a patchy basal lamina (Fig. 4C) (Gamble and Eames, 1964; Parmantier et al., 1999). A similar phenotype has been reported in Dhh–/– mice and has also been described in a human patient with a DHH mutation. In these cases, minifascicles are present throughout the endoneurium, much like Gpr126–/– nerves. The Dhh and Gpr126 mutant phenotypes differ, however, in that Dhh mutant Schwann cells are able to myelinate axons (Parmantier et al., 1999; Umehara et al., 2000). We did not observe Dhh mRNA expression in Gpr126–/– nerves (Fig. 1C), supporting the possibility that Gpr126 is involved in the regulation of Dhh expression in Schwann cells, together with other genes that drive the myelination program. Together, our ultrastructural analyses show that Gpr126 is essential for several key aspects of peripheral nerve development in mammals, including progression of Schwann cells beyond the promyelinating stage, axonal sorting, maintenance of axons, formation of Remak bundles and normal perineurium formation. Abnormalities in the auditory nerve in Gpr126–/– mutants The auditory nerve carries information from the sensory hair cells of the cochlea to the cochlear nucleus in the brainstem. The afferent auditory neuron cell bodies are located in the spiral ganglion in the central stalk of the cochlea. Peripheral myelination of the afferent fibers is mediated by Schwann cells and reaches from the habenula Fig. 3. Numerous ultrastructural abnormalities are observed in Gpr126–/– sciatic nerves. (A-H)Transmission electron micrographs showing ultrastructure of sciatic nerve in wild-type (+/+) and Gpr126+/– (+/–; B-D) or Gpr126–/– (–/–; E-H) animals at the indicated stages. (A)In +/+ sciatic nerve at P1, many axons are ensheathed by promyelinating Schwann cells (p) and a few axons are surrounded by a thin myelin sheath (m). (E)In –/– sciatic nerve at P1, axonal sorting is delayed and Schwann cells (s) are predominately found on the edge of axon bundles (a). (B)Several axons are myelinated (m) in +/– sciatic nerve at P4. (F)In –/– sciatic nerve at P4, axonal sorting remains delayed and minifascicles (arrow) including axons and Schwann cells (s) are observed. (C,D)Many axons are myelinated (m) in +/– sciatic nerve at P12 and myelin sheath thickness increases. Additionally, developing Remak bundles (R) are observed. (G,H)Axons (a) are sorted by Schwann cells (s) in –/– sciatic nerve at P12, no myelin is observed and minifascicles including axons and Schwann cells are readily observed (arrow in G). Scale bars: 2m in all panels. DEVELOPMENT 2676 RESEARCH ARTICLE Gpr126 is essential for PNS myelination RESEARCH ARTICLE 2677 Fig. 4. Perineurial fibroblasts are found throughout Gpr126–/– sciatic nerve and endoneurial collagen fibrils are disrupted. (A)Myelinated axons (black asterisks) and developing Remak bundles (white asterisks) are observed in wild-type and Gpr126+/–(+/–) nerve at P12. Fibroblasts are never observed to bundle axons and Schwann cells into minifascicles. (B)Minifascicles (outlined in white) are observed throughout the endoneurium of –/– sciatic nerve. (C)The minifascicles are formed by perineurial fibroblasts, identified by numerous caveolae (arrowheads) and a patchy basal lamina (arrow). (D)Schwann cells in wild-type and +/– nerve are surrounded by a basal lamina (arrowheads), and collagen fibrils are well organized (arrow). (E)Collagen fibrils are often poorly organized in –/– sciatic nerve (arrow), although Gpr126–/– Schwann cells are surrounded by a basal lamina (arrowheads). (A,B)Scale bars: 5m in A,B; 0.5m in C-E. consistently expanded deeper into the modiolus of Gpr126–/– nerves (Fig. 5B,D). At the glial limitans, where the transition between central and peripheral myelin occurs (Hurley et al., 2007), we observed many central MBP-positive cells projecting farther into the peripheral region of Gpr126–/– nerves than in WT nerves (Fig. 5A,B,D). Similar expansions of CNS myelin into the PNS have recently been reported in the absence of peripheral glia in zebrafish (Kucenas et al., 2009) and in mammals when Schwann cells or Egr2 function are absent (Coulpier et al., 2010). Together, this analysis shows that Schwann cells do not express MBP or P0 in Gpr126–/– nerves, and that Gpr126–/– oligodendrocytes express MBP and project aberrantly into PNS regions of the cochlear nerve in the absence of peripheral myelin but in the presence of Gpr126–/– Schwann cells. DISCUSSION Beginning with a forward genetic screen for mutations disrupting the development of myelinated axons, we recently described an essential role for Gpr126 in Schwann cell myelination in zebrafish (Monk et al., 2009). Through analysis of Gpr126–/– mice, we show that the function of Gpr126 in myelination is conserved in mammalian peripheral nerve: Schwann cells are arrested at the promyelinating stage in Gpr126–/– mice, as in zebrafish. This myelination defect is specific to the PNS, as CNS myelination appears to be normal in Gpr126–/– mice (Fig. 5; see Fig. S2 in the supplementary material). In addition, this study defines new functions of Gpr126 by analyzing other defects in Gpr126–/– mice that were not evident in gpr126 zebrafish mutants. The analysis of peripheral nerves in Gpr126 mutant mice defines a previously unknown function for Gpr126 in the organization of the perineurium. By P4 in Gpr126–/– mutants, the ectopic localization of apparent perineurial fibroblasts is observed throughout the endoneurium (Fig. 3F). This phenotype worsens with age and, by P12, the endoneurium of Gpr126–/– mutants is partitioned into numerous distinct minifascicles by the fibroblasts (Fig. 3G, Fig. 4B). This defect is likely to be due to loss of Gpr126 in Schwann cells and not in fibroblasts: loss of desert hedgehog (Dhh) in Schwann cells also leads to ectopic fascicle formation by DEVELOPMENT perforata, which demarks the entry point of the afferent fibers into the organ of Corti. Schwann cell myelination continues until the central-peripheral transitional zone, also known as the glial limitans, which is located towards the base of the cochlear modiolus (Fraher and Delanty, 1987). Central myelination, mediated by oligodendrocytes, stretches from the glial limitans to the cochlear nucleus, the first central echelon of the auditory pathway. We previously reported that gpr126 mutant zebrafish larvae have visibly swollen ears but did not pursue further phenotypic analyses (Monk et al., 2009). To investigate the possibility that Gpr126 is required for development of the auditory system in mammals, we examined inner ear morphology and various markers in mid-modiolar cochlear sections at P14. No obvious defects were observed in ear or cochlea morphology upon gross examination, and hair cell morphology and innervation appeared normal as assessed by neurofilament-M immunostaining in Gpr126–/– animals compared with WT siblings (Fig. 5A; data not shown). We also examined myelin protein expression in the centralperipheral transitional zone of the auditory nerve in mid-modiolar cochlear sections at P14. In WT mice, myelin basic protein (MBP) was evident in both the CNS and the PNS regions of the nerve, as assessed by immunostaining (Fig. 5). In the peripheral portion of the auditory nerve of WT and Gpr126+/– mice, MBP was colocalized with myelin protein zero (P0), a major structural component of Schwann cell, but not oligodendrocyte, myelin (Lemke et al., 1988). MBP was strongly expressed in CNS regions of Gpr126–/– cochlear nerve (Fig. 5), providing further evidence that oligodendrocytes can form myelin in the absence of Gpr126 function. In the PNS region of the nerve, however, no P0 or MBP expression was detected in Gpr126–/– mutants, specifically in the spiral ganglia and in peripheral neurites between the spiral ganglion and the habenula perforata, where the fibers enter the organ of Corti (Fig. 5B,C). Schwann cell numbers in these regions did not appear to be disrupted, as shown by DAPI staining (Fig. 5C). Interestingly, the part of the auditory nerve known as the central tissue projection (Fraher and Delanty, 1987), which is myelinated by oligodendrocytes and strongly labeled with MBP antibody, was 2678 RESEARCH ARTICLE Development 138 (13) Fig. 5. Cochlear histology appears grossly normal in Gpr126–/– mice, but Schwann cells do not express P0 or MBP, and CNS myelin is expanded into peripheral zones. (A)Mid-modiolar cross sections showing the middle turn of wild-type (+/+) and Gpr126–/– (–/–) mice, stained with antibodies to myelin basic protein (MBP; red) and neurofilament-M (NF-M; green). (B) Adjacent sections to those shown in A, labeled with antibodies to MBP (red) and peripheral myelin protein zero (P0; green). The brackets indicate the region between the habenula perforata and the spiral ganglion; the arrow indicates the spiral ganglion. (C)Higher magnification of the peripheral section of the auditory nerve fibers. The habenula perforata (hp) and the spiral ganglion (sg) are indicated. (D)Higher magnification of the glial limitans, where the transition between central and peripheral myelin occurs. Arrowhead indicates projections of MBP-positive CNS myelin into the peripheral region in –/– nerves. In A,B and D, asterisks indicate CNS myelin. Scale bars: 200m in A,B; 100m in C,D. Impaired radial sorting in Gpr126–/– nerves During embryonic nerve development, in a process known as radial sorting, Schwann cells associated with multiple axons interdigitate cytoplasmic extensions throughout the bundle to separate large caliber axons and eventually myelinate them. At P1 in Gpr126–/– nerve, Schwann cells are found mainly at the periphery of axon bundles, and very few axons have been sorted (Fig. 3E). Similar defects in radial sorting are found in peripheral nerves of mice with Schwann cell-specific mutations affecting laminin isoforms (Chen and Strickland, 2003; Yu et al., 2005), the laminin receptor b1integrin (Feltri et al., 2002; Nodari et al., 2007), the tyrosine kinase FAK, integrin-linked kinase and downstream signaling components in Schwann cells (Benninger et al., 2006; Grove et al., 2007; Nodari et al., 2007; Pereira et al., 2009). Although Schwann cells in Gpr126–/– mutant mice eventually recover and sort axons, these early phenotypic similarities suggest a possible connection between Gpr126 and signaling between laminins and their integrin receptors. It is also possible that activation of Gpr126 in Schwann cells leads to similar downstream signaling cascades as integrin activation. Reduced axon number in Gpr126–/– nerves The total number of axons in Gpr126–/– sciatic nerve was substantially reduced compared with the estimated total number of axons in WT sciatic nerve at P8. However, at P1, this reduction was less severe. Additionally, when we viewed the entire sciatic nerve by electron microscopy montage at P1, we observed regions of mutant nerve with dying axons (data not shown). In ErbB3 mutant mice, which lack Schwann cells associated with developing sensory and motor neurons, loss of trophic support from Schwann cells results in increased neuronal death in the embryo (Riethmacher et al., 1997). Schwann cells are present in Gpr126–/– mutants, but our results suggest that Schwann cells lacking Gpr126 cannot confer vital trophic support to associated axons, resulting in the loss of axons we observe at P8. Absence of Remak bundles in Gpr126–/– nerves We observed no developing Remak bundles in Gpr126–/– sciatic nerves as late as P12. Axonal sorting was initially delayed, and Schwann cells eventually sorted axons into a 1:1 relationship. Bundles of unsorted axons were occasionally observed at P12 (data DEVELOPMENT perineurial fibroblasts (Parmantier et al., 1999). These fibroblasts express the hedgehog receptor patched homolog 1 (Ptch1), and require Dhh from Schwann cells during nerve development for proper perineurium formation (Parmantier et al., 1999). Dhh is not expressed in Gpr126–/– sciatic nerve (Fig. 1C), suggesting that the minifascicle pathology found throughout the endoneurium of Gpr126–/– nerves is due to loss of Dhh in Schwann cells rather than a direct role for Gpr126 in fibroblast development. Gpr126–/– nerve pathology is also reminiscent of defects observed in Adam22 and Lgi4 mutants (Henry et al., 1991; Darbas et al., 2004; Sagane et al., 2005; Ozkaynak et al., 2010; Nishino et al., 2010). Adam22 is a receptor for Lgi4; Adam22 is expressed neuronally, and Lgi4 is secreted principally by Schwann cells in peripheral nerve (Sagane et al., 2008; Ozkaynak et al., 2010). In addition to reduced Schwann cell myelination, Adam22 and Lgi4 mutant peripheral nerves also possess minifascicle pathologies throughout the endoneurium (Henry et al., 1991; Darbas et al., 2004; Sagane et al., 2005; Ozkaynak et al., 2010). Axonal sorting is also delayed in Lgi4 mutants (Darbas et al., 2004). Although these phenotypic similarities raise the possibility that Gpr126 activation requires Adam22 and/or Lgi4, it is important to note that Adam22 and Lgi4 mutants express Pou3f1 (Ozkaynak et al., 2010; Darbas et al., 2004; Nishino et al., 2010), whereas Gpr126 mutants do not (Fig. 2B) (Monk et al., 2009). Future studies are required to examine the possible interaction between Gpr126 and Adam22 and Lgi4. not shown), but these axons were not surrounded by nonmyelinating Schwann cell cytoplasm; rather, Schwann cells remained at the periphery of axon bundles, reminiscent of the sorting defect we observed at earlier stages (Fig. 3E,F). Although the total number of axons is reduced in Gpr126–/– nerves, small caliber C-fibers are present in mutants (Fig. 2D). This result suggests Gpr126 plays a role in the development of nonmyelinating Schwann cells, leading to the loss of Remak bundles in Gpr126–/– mutants. Previous results suggest that Gpr126 functions to elevate cAMP levels in promyelinating Schwann cells prior to myelination (Monk et al., 2009). cAMP elevation in postmitotic Schwann cells is thought to downregulate premyelinating signals and upregulate myelinating signals (Morgan et al., 1991; Monuki et al., 1989; Scherer et al., 1994). It is possible that non-myelinating Schwann cells also require elevation of cAMP levels in order to differentiate. Indeed, when Schwann cells are proliferative, elevation of cAMP does not initiate myelin gene expression but instead induces expression of the cell surface lipid antigen O4, an early Schwann cell differentiation marker (Mirsky et al., 1990; Morgan et al., 1991; Jessen et al., 1991). The levels of Nrg1TypeIII on the surface of an axon are thought to instruct Schwann cells to become myelinating or non-myelinating (Taveggia et al., 2005). It was recently shown that levels of cAMP determine how Schwann cells respond to Nrg1 in vitro (ArthurFarraj et al., 2011). Future studies are required to determine the role of cAMP in non-myelinating Schwann cell development and to ascertain how Gpr126 and Nrg signaling interact in vivo. It is also possible that non-myelinating Schwann cells require a signal from promyelinating or myelinating Schwann cells that is altered in the absence of Gpr126; one such candidate signaling molecule is Dhh. Non-myelinating Schwann cells express the hedgehog receptor patched homolog 2 (Ptch2) in adult sciatic nerve and after injury (Bajestan et al., 2006). Although Dhh-Ptch2 signaling has not been directly shown to be required for the development of non-myelinating Schwann cells, Dhh mutant nerves possess significantly more axons sorted in a 1:1 relationship and fewer axons in Remak bundles (Sharghi-Namini et al., 2006), a pathology that is reminiscent of the defects observed in Gpr126–/– peripheral nerve. Future studies utilizing cell-type specific ablation of Gpr126 will be useful to determine the role of Gpr126 in non-myelinating Schwann cell development. Other roles for Gpr126 and future directions Zebrafish mutants for gpr126 are viable and fertile, whereas Gpr126–/– mice are not born in Mendelian ratios and no mutant survived past P16. In a recent study, loss of Gpr126 in mouse resulted in mid-gestation embryonic lethality (Waller-Evans et al., 2010). These embryos died of cardiovascular defects before analysis of peripheral myelination could take place. It is therefore likely that cardiovascular defects also account for some of the embryonic lethality we observe in our studies. Strain differences or the region of Gpr126 targeted might explain why we obtained some live mutants at birth and why Waller-Evans et al. observed fully penetrant embryonic lethality. Waller-Evans et al. deleted the seven-transmembrane region of Gpr126 in their mutant allele, whereas functional domains in the N-terminus were targeted in the Gpr126 mutation that we analyzed. Gpr126 is also highly expressed in mouse and rat lungs (Moriguchi et al., 2004; Haitina et al., 2008) so it is possible that an abnormality in the lung contributes to the mortality of Gpr126 mutants. Furthermore, three single-nucleotide polymorphisms (SNPs) in GPR126 were recently associated with lung function in humans (Hancock et al., 2010). RESEARCH ARTICLE 2679 Bergmann glial cells of the mouse cerebellum also express high levels of Gpr126 (Koirala and Corfas, 2010), as does the collecting duct of the mouse kidney (Pradervand et al., 2010). Future studies are required to determine the function of Gpr126 in these tissues and define the cause of postnatal lethality in the mutant mice. Peripheral nerves are complex structures containing axons, Schwann cells, fibroblasts and other cell types. Precise signaling between these cells is required for the proper development, organization and function of peripheral nerves. This work shows that Gpr126 is essential for myelination in the mammalian PNS. We also show that Gpr126 is required for timely Schwann cell differentiation and peripheral nerve organization in mice. Many key drug targets are G protein-coupled receptors (Lundstrom, 2005); as a crucial regulator of myelination in mammals, Gpr126 might represent an attractive therapeutic target for re-myelination therapy in humans. Acknowledgements This work was supported by grants to W.S.T. from the Muscular Dystrophy Association (92233) and the National Institutes of Health (NIH; R01 NS050223). K.R.M. was supported by a National Multiple Sclerosis Society postdoctoral award (FG 1719-A-1) and by the Stanford Genome Training Program (NHI/NGHRI). S.H. was supported by NIH R01 DC004563 and P30 DC010363. We thank Ben Emery for assistance with optic nerve dissections, John Perrino for electron microscopy advice, the Barres lab for the gift of the P0 antibody, and members of the Talbot and Kingsley laboratories for helpful discussions. We are also grateful to Yo Sasaki and Jeffrey Milbrandt for providing DRG neuron cDNA samples, and to Valeria Cavalli, John Heuser, David Ornitz, Naren Ramanan and Lila Solnica-Krezel for sharing laboratory space and reagents. Deposited in PMC for release after 12 months. Competing interests statement The authors declare no competing financial interests. Supplementary material Supplementary material for this article is available at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.062224/-/DC1 References Arthur-Farraj, P., Wanek, K., Hantke, J., Davis, C. M., Jayakar, A., Parkinson, D. B., Mirsky, R. and Jessen, K. R. (2011). Mouse Schwann cells need both NRG1 and cyclic AMP to myelinate. Glia 59, 720-733. Bajestan, S. N., Umehara, F., Shirahama, Y., Itoh, K., Sharghi-Namini, S., Jessen, K. R., Mirsky, R. and Osame, M. (2006). Desert hedgehog-patched 2 expression in peripheral nerves during Wallerian degeneration and regeneration. J. Neurobiol. 66, 243-255. Bamshad, M., Van Heest, A. E. and Pleasure, D. (2009). Arthrogryposis: a review and update. J. Bone Joint Surg. Am. 91, 40-46. Benninger, Y., Colognato, H., Thurnherr, T., Franklin, R. J., Leone, D. P., Atanasoski, S., Nave, K. A., Ffrench-Constant, C., Suter, U. and Revlas, J. B. (2006). Beta1-integrin signaling mediates premyelinating oligodendrocyte survival but is not required for CNS myelination and remyelination. J. Neurosci. 26, 7665-7673. Bermingham, J. R., Jr, Scherer, S. S., O’Connell, S., Arroyo, E., Kalla, K. A., Powell, F. L. and Rosenfeld, M. G. (1996). Tst-1/Oct-6/SCIP regulates a unique step in peripheral myelination and is required for normal respiration. Genes Dev. 10, 1751-1762. Bermingham, J. R., Jr, Shearin, H., Pennington, J., O’Moore, J., Jaegle, M., Driegen, S., van Zon, A., Darbas, A., Ozkaynak, E., Ryu, E. J. et al. (2006). The claw paw mutation reveals a role for Lgi4 in peripheral nerve development. Nat. Neurosci. 9, 76-84. Britsch, S., Goerich, D. E., Riethmacher, D., Peirano, R. I., Rossner, M., Nave, K. A., Birchmeier, C. and Wegner, M. (2001). The transcription factor Sox10 is a key regulator of peripheral glial development. Genes Dev. 15, 66-78. Chen, Z. L. and Strickland, S. (2003). Laminin gamma1 is critical for Schwann cell differentiation, axon myelination, and regeneration in the peripheral nerve. J. Cell Biol. 163, 889-899. Coulpier, F., Decker, L., Funalot, B., Vallat, J. M., Garcia-Bragado, F., Charnay, P. and Topilko, P. (2010). CNS/PNS boundary transgression by central glia in the absence of Schwann cells or Krox20/Egr2 function. J. Neurosci. 30, 5958-5967. Darbas, A., Jaegle, M., Walbeehm, E., van den Burg, H., Driegen, S., Broos, L., Uyl, M., Visser, P., Grosveld, F. and Meijer, D. (2004). Cell autonomy of the mouse claw paw mutation. Dev. Biol. 272, 470-482. Feltri, M. L., Graus Porta, D., Previtali, S. C., Nodari, A., Migliavacca, B., Cassetti, A., Littlewood-Evans, A., Reichardt, L. F., Messing, A., Quattrini, A. DEVELOPMENT Gpr126 is essential for PNS myelination et al. (2002). Conditional disruption of beta 1 integrin in Schwann cells impedes interactions with axons. J. Cell Biol. 156, 199-209. Fraher, J. P. and Delanty, F. J. (1987). The development of the central-peripheral transitional zone of the rat cochlear nerve. A light microscopic study. J. Anat. 155, 109-118. Gamble, H. J. and Eames, R. A. (1964). An electron microscope study of the connective tissues of human peripheral nerve. J. Anat. 98, 655-663. Garratt, A. N., Voiculescu, O., Topilko, P., Charnay, P. and Birchmeier, C. (2000). A dual role of erbB2 in myelination and in expansion of the Schwann cell precursor pool. J. Cell Biol. 148, 1035-1046. Goldstein, M. E., House, S. B. and Gainer, H. (1991). NF-L and peripherin immunoreactivities define distinct classes of rat sensory ganglion cells. J. Neurosci. Res. 30, 92-104. Grove, M., Komiyama, N. H., Nave, K. A., Grant, S. G., Sherman, D. L. and Brophy, P. J. (2007). Fak is required for axonal sorting by Schwann cells. J. Cell Biol. 176, 277-282. Haitina, T., Olsson, F., Stephansson, O., Alsio, J., Roman, E., Ebendal, T., Schioth, H. B. and Fredriksson, R. (2008). Expression profile of the entire family of Adhesion G protein-coupled receptors in mouse and rat. BMC Neurosci. 9, 43. Hancock, D. B., Eijgelsheim, M., Wilk, J. B., Gharib, S. A., Loehr, L. R., Marciante, K. D., Franceschini, N., van Durme, Y. M., Chen, T. H., Barr, R. G. et al. (2010). Meta-analyses of genome-wide association studies identify multiple loci associated with pulmonary function. Nat. Genet. 42, 45-52. Henry, E. W., Eicher, E. M. and Sidman, R. L. (1991). The mouse mutation claw paw: forelimb deformity and delayed myelination throughout the peripheral nervous system. J. Hered. 82, 287-294. Hurley, P. A., Crook, J. M. and Shepherd, R. K. (2007). Schwann cells revert to nonmyelinating phenotypes in the deafened rat cochlea. Eur. J. Neurosci. 26, 1813-1821. Jaegle, M., Mandemakers, W., Broos, L., Zwart, R., Karis, A., Visser, P., Grosveld, F. and Meijer, D. (1996). The POU factor Oct-6 and Schwann cell differentiation. Science 273, 507-510. Jaegle, M., Ghazvini, M., Mandemakers, W., Piirsoo, M., Driegen, S., Levavasseur, F., Raghoenath, S., Grosveld, F. and Meijer, D. (2003). The POU proteins Brn-2 and Oct-6 share important functions in Schwann cell development. Genes Dev. 17, 1380-1391. Jessen, K. R. and Mirsky, R. (2002). Signals that determine Schwann cell identity. J. Anat. 200, 367-376. Jessen, K. R. and Mirsky, R. (2005). The origin and development of glial cells in peripheral nerves. Nat. Rev. Neurosci. 6, 671-682. Jessen, K. R., Mirsky, R. and Morgan, L. (1991). Role of cyclic AMP and proliferation controls in Schwann cell differentiation. Ann. N. Y. Acad. Sci. 633, 7889. Koirala, S. and Corfas, G. (2010). Identification of novel genes by single-cell transcriptional profiling of Bergmann glial cells from mouse cerebellum. PLoS ONE 5, e9198. Kucenas, S., Wang, W. D., Knapik, E. W. and Appel, B. (2009). A selective glial barrier at motor axon exit points prevents oligodendrocyte migration from the spinal cord. J. Neurosci. 29, 15187-15194. Kuhlbrodt, K., Herbarth, B., Sock, E., Hermans-Borgmeyer, I. and Wegner, M. (1998). Sox10, a novel transcriptional modulator in glial cells. J. Neurosci. 8, 237250. Lemke, G., Lamar, E. and Patterson, J. (1988). Isolation and analysis of the gene encoding peripheral myelin protein zero. Neuron 1, 73-83. Lundstrom, K. (2005). Structural genomics of GPCRs. Trends Biotechnol. 23, 103108. Michailov, G. V., Sereda, M. W., Brinkmann, B. G., Fischer, T. M., Haug, B., Birchmeier, C., Role, L., Lai, C., Schwab, M. H. and Nave, K. (2004). Axonal neuregulin-1 regulates myelin sheath thickness. Science 304, 700-703. Mirsky, R., Dubois, C., Morgan, L. and Jessen, K. R. (1990). 04 and A007sulfatide antibodies bind to embryonic Schwann cells prior to the appearance of galactocerbroside: regulation by axon-Schwann cell signals and cyclic AMP. Development 109, 105-116. Monk, K. R., Naylor, S. G., Glenn, T. D., Mercurio, S., Perlin, J. R., Dominguez, C., Moens, C. B. and Talbot, W. S. (2009). A G protein-coupled receptor is essential for Schwann cells to initiate myelination. Science 325, 1402-1405. Monuki, E. S., Weinmaster, G., Kuhn, R. and Lemke, G. (1989). SCIP: a glial POU domain gene regulated by cyclic AMP. Neuron 3, 783-793. Morgan, L., Jessen, K. R. and Mirsky, R. (1991). The effects of cAMP on differentiation of cultured Schwann cells: progression from an early phenotype (04+) to a myelin phenotype (P0+, GFAP–, N-CAM–, NGF-Receptor–) depends on growth inhibition. J. Cell Biol. 112, 457-467. Moriguchi, T., Haraguchi, K., Ueda, N., Okada, M., Furuya, T. and Akiyama, T. (2004). DREG, a developmentally regulated G protein-coupled receptor containing two conserved proteolytic cleavage sites. Genes Cells 9, 549-560. Nave, K. A. and Salzer, J. L. (2006). Axonal regulation of myelination by neuregulin 1. Curr. Opin. Neurobiol. 16, 492-500. Development 138 (13) Nishino, J., Saunders, T. L., Sagane, K. and Morrison, S. J. (2010). Lgi4 promotes the proliferation and differentiation of glial lineage cells throughout the development peripheral nervous system. J. Neurosci. 30, 15228-15240. Nodari, A., Zambroni, D., Quattrini, A., Court, F. A., D’Urso, A., Recchia, A., Tybulewicz, V. L., Wrabetz, L. and Feltri, M. L. (2007). Beta1 integrin activates Rac1 in Schwann cells to generate radial lamellae during axonal sorting and myelination. J. Cell Biol. 177, 1063-1075. Oshima, K., Senn, P. and Heller, S. (2009). Isolation of sphere-forming stem cells from the mouse inner ear. Methods Mol. Biol. 493, 141-162. Ozkaynak, E., Abello, G., Jaegle, M., van Berge, L., Hamer, D., Kegel, L., Driegen, S., Sagane, K., Bermingham, J. R., Jr and Meijer, D. (2010). Adam22 is a major neuronal receptor for Lgi4-mediated Schwann cell signaling. J. Neurosci. 30, 3857-3864. Parmantier, E., Lynn, B., Lawson, D., Turmaine, M., Namini, S. S., Chakrabarti, L., McMahon, A. P., Jessen, K. R. and Mirsky, R. (1999). Schwann cell-derived Desert hedgehog controls the development of peripheral nerve sheaths. Neuron 23, 713-724. Peirano, R. I., Goerich, D. E., Riethmacher, D. and Wegner, M. (2000). Protein zero gene expression is regulated by the glial transcription factor Sox10. Mol. Cell. Biol. 20, 3198-3209. Pereira, J. A., Benninger, Y., Baumann, R., Gonçalves, A. F., Ozçelik, M., Thurnherr, T., Tricaud, N., Meijer, D., Fässler, R., Suter, U. et al. (2009). Integrin-linked kinase is required for radial sorting of axons and Schwann cell remyelination in the peripheral nervous system. J. Cell Biol. 185, 147-161. Pogoda, H. M., Sternheim, N., Lyons, D. A., Diamond, B., Hawkins, T. A., Woods, I. G., Bhatt, D. H., Franzini-Armstrong, C., Dominguez, C., Arana, N. et al. (2006). A genetic screen identifies genes essential for development of myelinated axons in zebrafish. Dev. Biol. 298, 118-131. Pradervand, S., Zuber Mercier, A., Centeno, G., Bonny, O. and Firsov, D. (2010). A comprehensive analysis of gene expression profiles in distal parts of the mouse renal tubule. Pflugers Arch. 460, 925-952. Riethmacher, D., Sonnenberg-Riethmacher, E., Brinkmann, V., Yamaai, T., Lewin, G. R. and Birchmeier, C. (1997). Severe neuropathies in mice with targeted mutations in the ErbB3 receptor. Nature 389, 725-730. Sagane, K., Yamazaki, K., Kai, J., Hirohashi, T., Takahashi, E., Miyamoto, N., Ino, M., Oki, T., Yamazaki, K. and Nagasu, T. (2005). Ataxia and peripheral nerve hypomyelination in ADAM22-deficient mice. BMC Neurosci. 6, 33. Sagane, K., Ishihama, Y. and Sugimoto, H. (2008). LGI1 and LGI4 bind to ADAM22, ADAM23 and ADAM11. Int. J. Biol. Sci. 4, 387-396. Sasaki, Y., Vohra, B. P. S., Baloh, R. H. and Milbrandt, J. (2009). Transgenic mice expressing the Nmnat1 protein manifest robust delay in axonal degeneration in vivo. J. Neurosci. 29, 6526-6534. Scherer, S. S., Xu, Y. T., Roling, D., Wrabetz, L., Feltri, M. L. and Kamholz, J. (1994). Expression of growth-associated protein-43 kD in Schwann cells is regulated by axon-Schwann cell interactions and cAMP. J. Neurosci. Res. 38, 575589. Sharghi-Namini, S., Turmaine, M., Meier, C., Sahni, V., Umehara, F., Jessen, K. R. and Mirsky, R. (2006). The structural and functional integrity of peripheral nerves depends on the glial-derived signal desert hedgehog. J. Neurosci. 26, 63646376. Stehlik, C., Kroismayr, R., Dorfleutner, A., Binder, B. R. and Lipp, J. (2004). VIGR – a novel inducible adhesion family G-protein coupled receptor in endothelial cells. FEBS Lett. 569, 149-155. Svaren, J. and Meijer, D. (2008), The molecular machinery of myelin gene transcription in Schwann cells. Glia 56, 1541-1552. Taveggia, C., Zanazzi, G., Petrylak, A., Yano, H., Rosenbluth, J., Einheber, S., Xu, X., Esper, R. M., Loeb, J. A., Shrager, P. et al. (2005). Neuregulin-1 type III determines the ensheathment fate of axons. Neuron 47, 681-694. Topilko, P., Schneider-Maunoury, S., Levi, G., Baron-Van Evercooren, A., Chennoufi, A. B., Seitanidou, T., Babinet, C. and Charnay, P. (1994). Krox-20 controls myelination in the peripheral nervous system. Nature 371, 796-799. Umehara, F., Tate, G., Itoh, K., Yamaguchi, N., Douchi, T., Mitsuya, T. and Osame, M. (2000). A novel mutation of desert hedgehog in a patient with 46,XY partial gonadal dysgenesis accompanied by minifascicular neuropathy. Am. J. Hum. Genet. 67, 1302-1305. Vargas, M. E., Watanabe, J., Singh, S. J., Robinson, W. H. and Barres, B. A. (2010). Endogenous antibodies promote rapid myelin clearance and effective axon regeneration after nerve injury. Proc. Natl. Acad. Sci. USA 107, 11993-11998. Waller-Evans, H., Prömel, S., Langenhan, T., Dixon, J., Zahn, D., Colledge, W. H., Doran, J., Carlton, M. B., Davies, B., Aparicio, S. A. et al. (2010). The orphan adhesion-GPCR Gpr126 is required for embryonic development in the mouse. Plos ONE 5, e14047. Yu, W. M., Feltri, M. L., Wrabetz, L., Strickland, S. and Chen, Z. L. (2005). Schwann cell-specific ablation of laminin gamma1 causes apoptosis and prevents proliferation. J. Neurosci. 25, 4463-4472. Zorick, T. S., Syroid, D. E., Brown, A., Gridley, T. and Lemke, G. (1999). Krox-20 controls SCIP expression, cell cycle exit and susceptibility to apoptosis in developing myelinating Schwann cells. Development 126, 1397-1406. DEVELOPMENT 2680 RESEARCH ARTICLE