* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Phenols Like alcohols, phenols are starting materials for a wide
Woodward–Hoffmann rules wikipedia , lookup
Marcus theory wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
George S. Hammond wikipedia , lookup
Organosulfur compounds wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Hydroformylation wikipedia , lookup
Aromaticity wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Stille reaction wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Homoaromaticity wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Aromatization wikipedia , lookup
Petasis reaction wikipedia , lookup
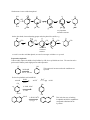
Phenols Like alcohols, phenols are starting materials for a wide range of compounds. The functional group is again OH but unlike alcohols, it is attached directly to a benzene ring and this affects its reactivity. Parent compound: OH phenol Nomenclature Named as derivatives of phenol OH OH OH CH3 NO2 O 2N Cl NO2 2-methylphenol o-methylphenyl o-cresol 2,4,6-trinitrophenol picric acid 4-chlorophenol p-chlorophenol Some compounds have 2 or more -OH groups, named as benzenediols: OH OH OH OH OH 1,2-benzenediol catechol OH Naphthols OH 1,4-benzenediol hydroquinone 1,3-benzenediol resorcinol OH 1-naphthol 2-naphthol Phenols are considerably more acidic than alcohols since the hydroxyl group is directly attached to the benzene ring. This allows resonance stabilization of the anion formed by loss of the hydroxyl proton. O O O O OH OH- Notice that the -ve charge can be delocalized into the benzene ring and can be written on the o and p positions relative to the original hydroxyl group. The carbanion is stabilized further by electron-withdrawing groups in these positions. Nitrobenzene is more acidic than phenol - OH O O OH- O O O - - NO2 N + O O N O + O N + O O - - N O+O - - - very important resonance structure On the other hand, electron donating groups make the phenol less acidic, e.g. O OH OO CH3 CH3 CH3 CH3 - OH- N +O O - O - CH3 - unfavored resonance structure o-cresol is a weaker acid than phenol; m-cresol is a stronger acid than o- or p-cresol Preparation of phenols Cannot readily replace the halide of aryl halides by -OH, since aryl halides are inert. The reaction can be performed in industry under high pressure and temperature: OH Cl This reaction is incorrect unless the conditions are specified 2M NaOH 300oC, 150 atm Fusion of arylsulfonic acid with KOH KOH ArSO3H ArO-K+ o 300 C CH3 e.g. 1) KOH, 300oC H3O+ ArOH CH3 2) H3O+ SO3 H OH SO3 H 1) KOH, 300oC 2) H3O + OH This is the best way of making 2-naphthol and from it naphthalene compounds substituted in the 2-position Phenols can be prepared from amines. We will see this reaction when we come to the study of amines. Industrial preparation of phenol + CH2 =CHCH3 H 3C H 3PO4 CH H 3C CH 3 O 2, 100oC CH 3 C OOH CH 3 C OOH H 3C cumene H 3O + CH 3 C OOH H + H 3C C CH 3 ~50o C cumene hydroperoxide Mechanism of the oxidation H 3C O H 3O+ 250oC Friedel Crafts type reaction OH H 3C CH 3 H 3C H 3C C O OH 2 C O -H 2O CH 3 H O CH 3 C OH H 2O Reactions of phenols Like alcohols, they readily form a sodium salt, but since the proton on the OH group of phenols is more acidic, the salt is formed using NaOH, not Na itself. However the salt is not formed with a weak base like Na2CO3 OH O -Na + 2 + H2 O + 2NaOH Phenols are very reactive towards electrophilic aromatic substitution OH OH e.g. OH 1 eq Br2 Br Br o H 2O/0 C CCl4 + Br2 but, fast Br Notice that the catalyst FeBr3 is not needed in this reaction Mechanism OH OH OH Br+ OH Br + OH -H+ + + + H Br Br OH H Br H Br very important resonance structure H Br Br Oxidation of Phenol OH O OH Na2Cr2 O7 /H2O SNCl2/H2O or (KSO3)2 NO/ H2 O Fremy's salt Potassium nitrodisulfonate Fremy's salt O benzoquinone Redox reaction important in cells O CH3 H3C O H3C O O OH hydroquinone ubiquinones are ubiquitous in nature and are involved in the electron transfer process in cells. n is between 1 and 10 (CH2CH=CCH2 )nH Many phenols are biosynthesized in plants e.g. OH CH3 C O H vanillin The irritants in poison ivy are phenols HO (CH2)4CH3 O tetrahydrocannabinol (marijuana)