* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Alcohols and Phenols
Survey
Document related concepts
Transcript
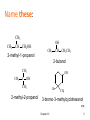
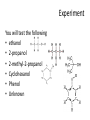
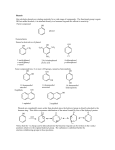
Alcohols and Phenols and how to identify them Classification • Primary: carbon with –OH is bonded to one other carbon • Secondary: carbon with –OH is bonded to two other carbons • Tertiary: carbon with –OH is bonded to three other carbons • Aromatic (phenol): -OH is bonded to a benzene ring 2 Classify these: CH3 CH3 CH3 CH CH2OH CH3 C OH CH3 OH OH CH3 CH CH2CH3 3 IUPAC Nomenclature • Find the longest carbon chain containing the carbon with the -OH group • Drop the -e from the alkane name, add –ol • Number the chain, starting from the end closest to the -OH group • Number and name all substituents. => 4 Name these: CH3 CH3 CH CH2OH 2-methyl-1-propanol OH CH3 CH CH2CH3 2-butanol CH3 CH3 OH C OH CH3 2-methyl-2-propanol Br CH3 3-bromo-3-methylcyclohexanol => Chapter 10 5 Naming Phenols • -OH group is assumed to be on carbon 1 • For common names of disubstituted phenols, use ortho- for 1,2; meta- for 1,3; and para- for 1,4 • Methyl phenols are cresols. OH Cl 3-chlorophenol meta-chlorophenol OH H3C 4-methylphenol para-cresol 6 Physical Properties • Unusually high boiling points due to hydrogen bonding between molecules • Small alcohols are miscible in water, but solubility decreases as the size of the alkyl group increases. 7 Acidity of Phenol • In water, phenol acts as a weak acid phenol phenoxide ion Oxidation of Alcohols: Do I have a tertiary alcohol? • • • For Primary alcohols that dehydrogenate from the aldehyde to the carboxylic acid tertiary alcohols do not dehydrogenate (oxidize) Secondary alcohols only oxidize to the ketone + Cr3+ + Cr3+ If chromic acid is used for H2CrO4=[O] then the product includes Cr3+ which is green 9 Oxidation with Chromate Negative test tertiary alcohol Positive test primary or secondary alcohol Test of a Phenol: FeCl3 test • You can test for a phenol with ferric chloride (FeCl3) Phenol + Fe3+ [Fe3+.phenol complex] colorless yellow purple Experiment You will test the following • ethanol • 2-propanol • 2-methyl-2-propanol • Cyclohexanol • Phenol • Unknown Experiment • • • • • • Odor Solubility in water Acidity Reaction with Chromic Acid Ferric Chloride Test Use test results to identify your unknown