* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Experiment 26 Bishop Voltaic and Electrolytic Cells Objective
Biochemical switches in the cell cycle wikipedia , lookup
Endomembrane system wikipedia , lookup
Cell encapsulation wikipedia , lookup
Extracellular matrix wikipedia , lookup
Programmed cell death wikipedia , lookup
Cellular differentiation wikipedia , lookup
Cell culture wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Cell growth wikipedia , lookup
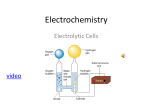
Experiment 26 Bishop Voltaic and Electrolytic Cells Objective: • Observe a Voltaic and Electrolytic Cells. • Determine where oxidation and reduction occurs in the different cells. • Calculate the Standard cell voltage from each electrode cell. Experiment: Part A. Voltaic Cell – Spontaneous Reduction Part B Electrolytic Cell – Non-Spontaneous Reduction Anode Oxidation Anion migrate to the anode Electrons leave the cell Negative in the voltaic cell Cathode Reduction Cations migrate to the cathode Electrode enters the cell Negative in the electrolytic cell. Procedure: Work in pairs. It is doable alone it just takes longer. Prepare your own U tube. You need to prepare a fresh one for each cell. • Dissolve 0.25 grams of agar in 25 mL of NaNO3 at 90o C. Don't boil it. • There must be no air bubbles at all. • To discard, heat the U tube and collect the melted agar in a test tube. Let it solidify before putting in the waste jar. Agar will clog the sinks. Voltmeter • Connect the leads according to color. • Black lead goes into the COM • Red lead goes into the V • Set the meter to V---, starting at 2 and adjusting as necessary. Part A: Check-out a voltmeter, U-tube Step 4. Iron II sulfate oxidizes readily and should be prepare immediately before use. To make a 1 M solution, dissolve 2.3 gram in 10 mL of distilled water. Step 8. Black is common and red goes to the DC voltage. The red terminal is positive. Wash the electrodes, wipe dry and remove oxidation by lightly sanding them. Return the electrodes to the original containers. Tin electrodes might be too thin to reuse - they can be put in the waste jar. Part B Check-out a power source, and electrode. Step 26. Use a 12 well plate. Step 27. Use well A1 A4 C1 C4 Step 30. Use power source instead of the 9 volt battery for the electrolytic cell ( Figure 26.6) Use the alligator clips to connect to the electrode. Set the voltage to 9. Adjust the polarity if voltage is negative. Step 33. After repeating the process with KI use starch pellet instead of starch solution. Step 34. Use a 100 mL beaker instead of a well plate.