* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Mutations in SIPA1L3 cause eye defects through disruption of cell
Signal transduction wikipedia , lookup
Tissue engineering wikipedia , lookup
Extracellular matrix wikipedia , lookup
Cell encapsulation wikipedia , lookup
Cell growth wikipedia , lookup
Cytokinesis wikipedia , lookup
Cell culture wikipedia , lookup
Cellular differentiation wikipedia , lookup
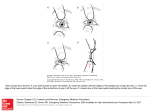
Human Molecular Genetics, 2015, Vol. 24, No. 20 5789–5804 doi: 10.1093/hmg/ddv298 Advance Access Publication Date: 30 July 2015 Original Article ORIGINAL ARTICLE Mutations in SIPA1L3 cause eye defects through disruption of cell polarity and cytoskeleton organization Rebecca Greenlees1,†, Marija Mihelec1,†, Saira Yousoof1,†, Daniel Speidel3, Selwin K. Wu6, Silke Rinkwitz2, Ivan Prokudin1, Rahat Perveen7, Anson Cheng1, Alan Ma1, Benjamin Nash1, Rachel Gillespie7, David A.F. Loebel4, Jill Clayton-Smith7,8, I. Christopher Lloyd7,8, John R. Grigg1, Patrick P.L. Tam4, Alpha S. Yap6, Thomas S. Becker2, Graeme C.M. Black7,8, Elena Semina5 and Robyn V. Jamieson1, * 1 Eye Genetics Research Group, Children’s Medical Research Institute, The University of Sydney; The Children’s Hospital at Westmead; and Save Sight Institute, Sydney, NSW, Australia, 2Brain and Mind Research Institute, Sydney Medical School, 3Cell Transformation Group, Children’s Medical Research Institute, Sydney Medical School 4Embryology Unit, Children’s Medical Research Institute, University of Sydney, Sydney, NSW, Australia, 5 Medical College of Wisconsin, Milwaukee, WI, USA, 6Division of Cell Biology and Molecular Medicine, Institute for Molecular Bioscience, The University of Queensland, Queensland, Australia, 7Manchester Centre for Genomic Medicine, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, Oxford Rd, Manchester, UK and 8Central Manchester Hospital NHS Foundation Trust, Manchester Academic Health Sciences Centre (MAHSC), St. Mary’s Hospital, Oxford Rd, Manchester, UK *To whom correspondence should be addressed at: Eye Genetics Research Group, Children’s Medical Research Institute, University of Sydney, Sydney, NSW 2145, Australia. Tel: +61 296872800; Fax: +61 296872120; Email: [email protected] Abstract Correct morphogenesis and differentiation are critical in development and maintenance of the lens, which is a classic model system for epithelial development and disease. Through germline genomic analyses in patients with lens and eye abnormalities, we discovered functional mutations in the Signal Induced Proliferation Associated 1 Like 3 (SIPA1L3) gene, which encodes a previously uncharacterized member of the Signal Induced Proliferation Associated 1 (SIPA1 or SPA1) family, with a role in Rap1 signalling. Patient 1, with a de novo balanced translocation, 46,XY,t(2;19)(q37.3;q13.1), had lens and ocular anterior segment abnormalities. Breakpoint mapping revealed transection of SIPA1L3 at 19q13.1 and reduced SIPA1L3 expression in patient lymphoblasts. SIPA1L3 downregulation in 3D cell culture revealed morphogenetic and cell polarity abnormalities. Decreased expression of Sipa1l3 in zebrafish and mouse caused severe lens and eye abnormalities. Sipa1l3−/− mice showed disrupted epithelial cell organization and polarity and, notably, abnormal epithelial to mesenchymal transition in the lens. Patient 2 with cataracts was heterozygous for a missense variant in SIPA1L3, c.442G>T, p.Asp148Tyr. Examination of the p.Asp148Tyr mutation in an epithelial cell line showed abnormal clustering of actin stress fibres and decreased formation of adherens junctions. Our findings show that abnormalities of SIPA1L3 in human, zebrafish and mouse contribute to lens and eye † The authors wish it to be known that, in their opinion, the first three authors should be regarded as joint First Authors. Received: April 2, 2015. Revised: June 23, 2015. Accepted: July 21, 2015 © The Author 2015. Published by Oxford University Press. All rights reserved. For Permissions, please email: [email protected] 5789 5790 | Human Molecular Genetics, 2015, Vol. 24, No. 20 defects, and we identify a critical role for SIPA1L3 in epithelial cell morphogenesis, polarity, adhesion and cytoskeletal organization. Introduction Normal epithelial cell development and function is reliant on a vast cascade of interacting factors required for cell adhesion, polarity and cytoskeletal organization. These processes are required for normal morphogenesis and prevention of abnormal epithelial cell behaviour. The vertebrate lens is a classic model system for development and disease (1) and provides an accessible structure for examination of the impact of novel factors contributing to epithelial cell function. As for many other tissues, the ocular lens requires correct epithelial cell–cell contacts and apical-basal polarity during the morphogenetic process and for maintenance of normal function. The lens placode is derived from epithelial cells of the surface ectoderm, which invaginate and separate from the surface ectoderm to form the lens vesicle. The anterior cells of the vesicle form a monolayer called the anterior epithelial layer of the lens, whereas the posterior cells elongate to fill the lumen of the vesicle. As the lens matures, the cells of the anterior layer at the equatorial region of the lens differentiate into lens fibre cells. Adhesive properties of the epithelial cells, cellular apical-basal polarity and cytoskeletal organization are required to maintain the structural properties of the cells and regulate cell differentiation and development for correct lens morphogenesis and maintenance (2,3). A variety of factors critical for cell adhesion, cell polarity and cellular structure in the lens are mutated in humans and mice with lens and developmental eye disorders, however not all are currently known. Lens and developmental eye disorders lead to blindness owing to congenital cataracts, corneal opacification, glaucoma and/or anophthalmia/microphthalmia (no/small eyes). These disorders cause a lifetime of visual disability and there is marked genetic heterogeneity with many of the pathogenic mechanisms not yet fully identified or understood (4) and no specific therapies are currently available. Genes where mutations are known to cause lens and eye developmental disorders include transcription factors PAX6, PITX3, FOXE3 and MAF (5–9), lens structural proteins such as connexins (10) and crystallins (11), cell adhesion and polarity proteins including cadherins (2) and aPKCλ (3), as well as components of the Rac1 signalling cascade, important in the regulation of cytoskeletal proteins (12). Tight regulation of the vast network of adhesion, polarity and cytoskeletal proteins is required for normal lens morphogenesis. Rap1, which has structural similarity to Rac1, is implicated in the regulation of cell adhesion, polarity and cytoskeletal function (13); however, the regulation of Rap1 controlled signalling or its role in the lens is not completely understood. The formation and maintenance of adherens and tight junctions for correct cellular adhesion is an active process that is known to be controlled by small guanine triphosphatase (GTPases), including Rap1. Rap1 is a member of the Ras superfamily of GTP-binding proteins that cycle between an active GTP- and inactive GDP-bound state. Tight regulation of Rap1 GTPase activity is imposed by guanine exchange factors that accelerate the GDP to GTP exchange and by GTPase-activating proteins (GAPs) that stimulate intrinsic GTP hydrolysis (14,15). GAPs specific for Rap1 include the mammalian Rap1GAP, tuberin (the tuberous sclerosis complex 2 product) and the SIPA1 or SPA1 (Signal Induced Proliferation Associated 1) family of genes (16–18). SIPA1 and SIPA1L (SIPA1-like) family members share a RapGAP and a PDZ domain. Members of the SIPA1L subfamily possess an additional C-terminal leucine zipper domain. In addition, SIPA1L1 harbours two regions of low homology found to associate with actin (19,20). Other members of the SIPA1L family include SIPA1L2 and SIPA1L3 (21,22). The expression of SIPA1L1 and SIPA1L2 (also called SPAR1 and 2) is enriched in hippocampal neurons and functional roles have been identified in shaping dendritic spine morphology (19,22,23). To date, there is a lack of information about the functional role of SIPA1L3. To explore novel factors critical in epithelial morphogenesis, we used patient, cell, zebrafish and mouse studies to identify and characterize the role of SIPA1L3 in lens epithelial cell function. Our investigation began with Patient 1, with cataracts and anterior segment abnormalities and a de novo balanced chromosomal translocation, where we revealed transection and reduced expression of SIPA1L3, leading to identification of SIPA1L3 as a candidate disease gene. We discovered that reduced SIPA1L3 expression in 3D cell culture led to defects in epithelial cell morphogenesis and polarity. This was recapitulated in the lens of our zebrafish and mouse mutants, where it was associated with abnormal epithelial to mesenchymal transition (EMT). We identified a second patient, Patient 2, with congenital cataracts and a heterozygous missense variant in SIPA1L3 in a region encoding a predicted actin-binding domain of the protein, with our cellular assay showing abnormal clustering of actin stress fibres in the presence of this mutation. Recent work (24), reported while this publication was in submission, identified a homozygous nonsense variant in SIPA1L3 (c.4489C>T, p.R1497X) in two siblings affected with congenital cataracts. This confirms our contention that SIPA1L3 is a causative disease gene affecting the lens. Our findings show that SIPA1L3 is a novel molecular factor contributing to epithelial cellular function, paving the way for improvements in our understanding of epithelial morphogenesis, polarity, adhesion and cytoskeletal organization. Results De novo translocation disrupted SIPA1L3 in a patient with lens and eye abnormalities We investigated Patient 1 who presented with bilateral severe ocular abnormalities including congenital cataracts, corneal clouding, iridocorneal and lenticular adhesions, and microphthalmia and also harboured a de novo balanced chromosomal translocation, 46,XY,t(2;19)(q37.3;q13.1) with unaffected parents (Fig. 1A and B). Chromosome microarray revealed no abnormalities, and next-generation sequencing (NGS) of known eye development disease genes revealed no pathogenic mutations (4). Breakpoint mapping and sequencing showed a physical disruption of the 5′ untranslated region of the gene, SIPA1L3 (NM_015073), on chromosome 19 (Fig. 1C,D, Supplementary Material, Fig. S1 and Table S1). The chromosomal breakpoints were within regions that did not contain genes previously implicated in developmental eye disorders. We considered SIPA1L3 the strongest candidate disease gene because: it was transected at the chromosome 19 breakpoint; we and others found the mouse orthologue showed strong expression in the developing lens [Fig. 1E and Lachke et al. (25)] in addition to a broad expression pattern (26); and the encoded protein had similarity to Human Molecular Genetics, 2015, Vol. 24, No. 20 | 5791 Figure 1. Transection and reduced expression of SIPA1L3 in a patient with lens abnormalities. (A) Patient 1 with a de novo balanced translocation, 46,XY,t(2;19)(q37.3;q13.1), exhibited bilateral microphthalmia and severe bilateral anterior segment dysgenesis characterized by sclerocornea, corneal-lens adhesion, cataracts and glaucoma. Anterior eye ultrasound in Patient 1 showed significantly reduced anterior chamber depth bilaterally. Arrows indicate the markedly narrowed anterior chamber. (B) The balanced chromosomal translocation identified in Patient 1 with anterior segment dysgenesis. The translocation affects the long arms of chromosomes 2 and 19 and resulted in derivative chromosomes, der (2) and der (19). Blue lines represent positions of chromosomal breakpoints, including a 19q13 transection that disrupted the 5′ untranslated region of SIPA1L3. (C) Base pair sequence shown in forward across the derivative chromosome 19 breakpoint. Chromatogram shows forward sequence of this region, with the blue line indicating the breakpoint. Normal chromosome 19 and chromosome 2 sequence is shown in this region with base pair numbering shown (Human Feb. 2009, GRCh37/hg19) with the blue letters indicating the derivative 19 breakpoint sequence on the respective normal chromosome 19 and 2 sequences. (D) Base pair sequence shown in forward across the derivative chromosome 2 breakpoint. Chromatogram shows reverse sequence of this region, with the blue line indicating the breakpoint. Normal chromosome 2 and chromosome 19 sequence are shown in this region with base pair numbering shown (Human Feb. 2009, GRCh37/hg19) with the blue letters indicating the derivative 2 breakpoint sequence on the respective normal chromosome 2 and 19 sequences. Grey letters indicate a 6-bp loss on chromosome 2. (E) In situ hybridization of WT mouse embryos at E10.5, E12.5 and E14.5 showing Sipa1l3 expression in the anterior lens epithelium (arrow) and lens fibres (arrowhead). E, embryonic day. (F) Quantitative PCR analysis of a lymphoblast cell line from Patient 1 with a balanced chromosomal translocation transecting the 5′ UTR of SIPA1L3. Derivative cell lines containing only the der2 and only the der19 chromosome were generated using mouse-human somatic cell hybrid lines. Patient 1 lymphoblast cell line showed a reduction in SIPA1L3 transcripts compared with control lymphoblast cell line. SIPA1L3 expression was not detected in der2 or der19 somatic cell lines. SIPA1 (SPA1) that is implicated in the regulation of Rap1 signalling (27), which is a candidate pathway for epithelial cell morphogenesis in the lens. There were no other genes 1 Mb either side of each of the breakpoints with a lens expression pattern or predicted function indicating strong candidate disease gene status (Supplementary Material, Tables S2 and S3). No other mutations in SIPA1L3 were identified in Patient 1. qPCR analysis of RNA extracted from lymphoblasts from Patient 1 revealed downregulation of SIPA1L3 (Fig. 1F), and the level of expression of SIPA1L3 in Patient 1 was <50% indicating possible allelic differences in the 5792 | Human Molecular Genetics, 2015, Vol. 24, No. 20 regulation of this gene (28,29). Expression was undetected in the derivative chromosomal translocation cell lines, and together these findings suggested the SIPA1L3 transection may cause or contribute to the patient phenotype through decreased expression of SIPA1L3. SIPA1L3 expression and knockdown in cellular culture revealed a crucial role in epithelial cell morphogenesis and polarity We sought to determine whether downregulation of SIPA1L3 may affect epithelial cell polarity and cytoskeletal function, two downstream effects of Rap1 signalling regulated by the SIPA1L3 homologue, SIPA1 (16,27,30–33) (Fig. 2A). We investigated cellular expression and functional impact of reduced expression of SIPA1L3 in two epithelial cell lines, colorectal adenocarcinoma (Caco2) cells and Madin Darby canine kidney (MDCK) cells. Cells expressing a construct containing SIPA1L3 conjugated to GFP (SIPA1L3+GFP) showed localization of GFP signals at the cell– cell borders in the apical regions (Fig. 2B–E; Supplementary Material, Fig. S2), tricellular junctions (Fig. 2F) and with apical F-actin (Fig. 2G). A 3D cell-based assay was performed using Caco2 cells, which form cysts in a 3D collagen matrix (34), to determine whether reduced SIPA1L3 expression affects epithelial morphogenesis. In Caco2 cells that were stably transduced with shRNA targeted to SIPA1L3 mRNA, SIPA1L3 expression was reduced by 70% (Fig. 3A). Knockdown cells formed significantly more cysts with ectopic lumens and multilayering of cells (Fig. 3D and E), compared with controls (Fig. 3B and C). Of the abnormal SIPA1L3 knockdown cells, one-third exhibited ectopic basal localization of atypical protein kinase C (aPKC) (Fig. 3E; P < 0.001), in addition to apical localization. This was not observed in untransduced Caco2 cysts and only in 2% of control shRNA cysts. The findings of abnormal epithelial morphogenesis and ectopic aPKC localization support a role of SIPA1L3 in epithelial morphogenesis and cell polarity. No consistent differences in E-cadherin, F-actin or ZO-1 staining were found in knockdown cysts. Reduced Sipa1l3 expression in zebrafish and mouse led to lens and anterior segment abnormalities and microphthalmia Our cell-based studies showed that reduced Sipa1l3 expression caused abnormal epithelial morphogenesis and cell polarity. We next determined whether disruption of Sipa1l3 function in zebrafish and mouse may impact on epithelial function and eye development. SIPA1L3 is well conserved across species (Supplementary Material, Fig. S3A), particularly within the functional domains (Supplementary Material, Fig. S3B,C), and its orthologue is expressed in the developing lens of zebrafish and mouse (Supplementary Material, Fig. S3D,E; Fig. 1E). Two morpholinos targeted to zebrafish sipa1l3 (ENDSART000088293, EnsEMBL release 59— August 2010) elicited abnormal splicing leading to loss of the predicted RapGAP- and PDZ-encoding regions of the gene (Fig. 4A,B; Supplementary Material, Fig. S3F). Sipa1l3 morphants exhibited a range of mild-to-severe ocular anomalies mimicking clinical features seen in the patients, including irregularly shaped and small lens and microphthalmia (Fig. 4C–F). We created a loss-of-function Sipa1l3 mouse line, using a gene trap ES cell line with a lacZ reporter integrated into the Sipa1l3 locus (35) (EUCOMM Consortium, Germany; Fig. 5A). LacZ staining revealed the expression of the gene trap allele in embryonic and adult Sipa1l3 −/− lens (Fig. 5B) in agreement with in situ hybridization data (Fig. 1E). qPCR analysis confirmed almost complete absence of Sipa1l3 expression in the lens of E18.5 Sipa1l3 −/− mutants compared with Sipa1l3 +/+ mice (Fig. 5C). Heterozygous Sipa1l3 +/− matings produced pups with normal Mendelian ratios on both 129/SvJ and C57BL/6 backgrounds (Table 1). Slit-lamp examination at 4 weeks postnatal age revealed reduced lens size and microphthalmia in Sipa1l3 −/− mutants. Cataracts were present in all Sipa1l3 −/− mice of both 129/SvJ and C57BL/6 backgrounds (Fig. 5D–I), and some Sipa1l3 −/− mice also exhibited lenticular-iridial adhesions (30% in C57BL/6 background) (Fig. 5G and Table 2). Histological analysis of the adult Sipa1l3 −/− eyes showed obvious abnormalities in the lens, with the presence of vacuoles and proteinaceous aggregates in place of lens fibre cells (Fig. 5J and K). We conclude that reduced expression of Sipa1l3 induced epithelial defects of the eye including lens and other anterior segment abnormalities in two vertebrate models. Sipa1l3−/− mouse lens exhibited abnormal cell adhesion, polarity and EMT Reduction of Sipa1l3 in the mouse demonstrated distinct abnormalities between the developing lens of wild-type (WT) and Sipa1l3 −/− mouse embryos, which were clearly seen at E18.5 (n = 5 per genotype). In Sipa1l3 −/− lens, we observed flattening and appearance of extra cells in the anterior lens epithelium (Fig. 6A–D), lack of distinctive equatorial zone (Fig. 6A,B,E and F), regions of vacuolation between the anterior lens epithelial cells and the adjacent posterior lens fibres (Fig. 6B, arrowhead) and abnormal cell clusters in the region of the expected germinative zone (Fig. 6E and F). At E14.5, there were no obvious additional cells in the anterior epithelium and the fibre cell mass appeared well-formed (Supplementary Material, Fig. S4A,B). In order to investigate possible cellular processes involved in cataract formation of Sipa1l3−/− mutants, we analysed the localization of proteins related to cellular polarity and cytoskeletal organization. At E18.5, Sipa1l3 −/− anterior lens epithelium exhibited an irregular pattern of E-cadherin and apical F-actin staining (Fig. 6H), in contrast with the well-ordered basal and lateral E-cadherin and apical F-actin staining in WT embryos (Fig. 6G). In Sipa1l3 −/− mutants, there was also disruption of ZO-1 and apical F-actin continuity in anterior lens epithelial cells (Fig. 6I and J) and marked reduction of ZO-1 staining of the epithelial cells at the lens equatorial region (Fig. 6K and L, arrow). Posteriorly, F-actin staining in mutant lens highlighted undulated lens fibre cells that frequently formed vacuoles throughout the lens at E18.5 (Fig. 6L). In addition, ZO-1 staining in the apical tips of the dislocated fibre cells appeared to lack uniformity compared with the normal lens (Fig. 6L, arrow). Similar morphological features were present in P10 and P21 mutants and at these ages, the lens was small with cataract formation underway (Supplementary Material, Fig. S4C–H, O–T). Abnormalities in E-cadherin, Factin and ZO-1 staining were more pronounced by P10 (Supplementary Material, Fig. S4I–N). Abnormalities of E-cadherin, F-actin, and ZO-1 staining in the epithelial cells of Sipa1l3 −/− embryos indicated loss of proper cytoskeletal organization, adhesion and polarity, which are processes that may be associated with EMT and cataract formation (36). The anterior epithelial cells of E18.5 Sipa1l3 −/− lens exhibited expression of alpha smooth muscle actin (αSMA), a marker of mesenchymal cells (Fig. 6N and P) and there were also abnormal cell clusters at the equatorial region (Fig. 6P). Neither phenotype was seen in WT lens (Fig. 6M and O). Overall, our findings suggest abnormalities in the fibre cells and anterior epithelial cells of the developing lens owing to polarity and organizational defects. In the anterior Human Molecular Genetics, 2015, Vol. 24, No. 20 | 5793 Figure 2. SIPA1L3 localization in epithelial cell lines. (A) The SPA1 (or SIPA1) family of related proteins showing the major conserved domains. RapGAP, PDZ, CC, CCLZ and GKBD domains were determined by in silico nucleotide sequence analysis. Actin-binding regions in SIPA1L1 (also known as SPAL1 or SPAR1) demonstrated actin-binding properties by functional analysis. SIPA1L2 and SIPA1L3 have also been known as SPAL2 and SPAL3 or SPAR2 and SPAR3. SIPA1L3 (NM_015073.1) consists of 22 exons, encoding a 1781-amino acid protein. Adapted from Spilker and Kreutz 2010 (23). Act1, actin-binding domain; CC, coiled-coiled; CCLZ, coiled-coiled leucine zipper; GKBD, guanylate kinase-binding domain; RapGAP, Rap1 GTPase-activating domain. (B–D) Apical sections of (B) GFP-transfected Caco2 cells, (C) SIPA1L3+GFPtransfected Caco2 cells and (D) MDCK cells transfected with SIPA1L3+GFP. SIPA1L3+GFP cells show plasma membrane localization of SIPA1L3 (arrows) that is not observed in the GFP only cells. Scale bar, 10 µm. (E–G) Co-staining of SIPA1L3 with epithelial markers in Caco2 cells on 2D culture. (E) Colocalization (arrow) at the lateral plasma membrane of SIPA1L3+GFP (green) and E-cadherin (red), an adherens junction marker. (F) Colocalization (arrow) of SIPA1L3+GFP (green) and ZO-1 (red), a tight junction marker. (G) Colocalization (arrow) of SIPA1L3+GFP (green) and F-actin labelled by phalloidin staining (red) at the apical F-actin belt (arrow). Scale bar, 10 µm. 5794 | Human Molecular Genetics, 2015, Vol. 24, No. 20 Figure 3. SIPA1L3 knockdown affects epithelial cell morphogenesis and polarity. (A) Relative expression levels of SIPA1L3 when compared with untransduced Caco2 cells. shRNAC shows a comparable level of SIPA1L3 to untransduced controls. The shRNA construct targeted for SIPA1L3 shows a 70% reduction of SIPA1L3 compared with untransduced and shRNAC. (B–D) Morphology and epithelial marker staining in Caco2 cysts grown in 3D culture showing abnormal cyst morphogenesis and ectopic aPKC localization (green) with shRNA knockdown of SIPA1L3. a, apical surface; b, basal surface. (B) Untransduced control and (C) shRNAC cysts show a central lumen with regular apical F-actin staining (red) and apical aPKC localization (green and arrowhead). (D) Cells transduced with SIPA1L3 knockdown vector (shRNA) showing central lumen with F-actin staining (red) and apical aPKC staining (green). Arrows indicate ectopic lumen; arrowheads indicate ectopic basal aPKC staining and multilayering of cells. Cell nuclei were stained with DAPI (blue). Scale bar, 20 µm. (E) Percentage of cysts (n = 50 per cell line) that were classified as normal or abnormal in the untransduced control, shRNAC, and shRNA SIPA1L3 knockdown cell lines. Abnormal cysts were classified as cysts that did not contain a monolayer of cells and/or exhibited aPKC staining in regions other than the apical central lumen. shRNA SIPA1L3 knockdown cell line produced a significantly greater number of abnormal cysts compared with all other lines *P < 0.05 by ANOVA. Abnormal cysts with ectopic aPKC staining were counted, with a significantly greater number in the shRNA cell line compared with controls (P < 0.001 by ANOVA). epithelial cells, EMT was observed, which may contribute to the cataract phenotype in the Sipa1l3 −/− lens. SIPA1L3 missense variant disrupted in vitro cytoskeletal organization and cell adhesion We sought SIPA1L3 variants in 80 other patients with lens abnormalities, other eye anterior segment defects or microphthalmia. Whole exome sequencing revealed a heterozygous, predicted pathogenic missense variant in SIPA1L3, c.442G>T (NM_015073) in Patient 2 with bilateral congenital cataracts. Unfortunately, parental samples were not available for this patient. The c.442G>T variant predicted an amino acid change, p.Asp148Tyr ( p.D148Y), in the first putative actin-binding domain of SIPA1L3 (Fig. 7A). The aspartate in this region is highly conserved across species and in silico analysis using Sorting Intolerant from Tolerant (SIFT) (37), MutationTaster (38) and PolyPhen-2 (39) predicted this mutation to be deleterious, with a minimum allele frequency of 0.000538 (ESP6500) (40). In view of decreased expression and predicted variant pathogenicity in SIPA1L3 in patients with abnormalities of the eye and lens, we investigated the functional consequence of the variant p.Asp148Tyr (p.D148Y) in epithelial cell culture. Caco2 cells transfected with normal SIPA1L3+GFP, in addition to the localization of the GFP-tagged protein at the cell–cell Human Molecular Genetics, 2015, Vol. 24, No. 20 | 5795 Figure 4. Reduced expression of Sipa1l3 in zebrafish leads to abnormal lens development, coloboma and microphthalmia. (A) Zebrafish sipa1l3 (20 exons) targeted by two antisense morpholinos, sipa1l3 ex2/3 MO1 and sipa1l3 ex4/5 MO2 (red bars) to the splice donor sites of exon 2–intron 2 and exon 4–intron 4, respectively. Forward and reverse primers (arrows) were designed across exons 1 and 3 for MO1 and exons 3 and 5 for MO2, respectively. PDZ- and RapGAP-encoding regions are indicated. (B) RT-PCR analysis of sipa1l3 transcripts in MO1- and MO2-injected embryos. Splice inhibition by MO1 and MO2 produced abnormal mRNA transcripts of 384 bp and 285 bp, in these regions, respectively. (C–E) Phenotype of WT and sipa1l3 morphant zebrafish embryos at 5 dpf. (C) Control morphant shows normal eye size, lens and optic fissure development. (D) sipa1l3 morphant (MO1) displays an abnormal lens (arrowhead) and ‘mild’ coloboma (arrow). (E) sipa1l3 morphant (MO2) displays microphthalmia, an irregularly shaped lens (arrowhead) and ‘severe’ coloboma (arrow). (F) Analysis of morphological phenotypes in morpholino-mediated knockdown of sipa1l3 in zebrafish showed that MO1 and MO2 produced phenotypes which included abnormal lens and microphthalmia that were more frequent (***P < 0.001) than in the standard MO injected and uninjected embryos. Increased concentration of injected morpholino resulted in more severe eye abnormalities. Mild and severe phenotypes were categorized according to appearances (see b and c). Light grey bars represent lethality. borders, tricellular junctions and apical F-actin (Fig. 3B and C), also showed expression in a typical actin stress fibre pattern in the basal cellular regions (Fig. 7F–I). In contrast, those transfected with the missense variant (D148Y+GFP) exhibited marked abnormal clustering of D148Y+GFP and F-actin staining in the basal part of the cell (Fig. 7J–M, arrowheads), whereas untransfected cells (Fig. 7K, arrows) and cells transfected with a control GFP vector or SIPA1L3+GFP showed a normal actin fibre distribution (Fig. 7B–I, arrows). Quantification of cell fractions in D148Y +GFP-transfected Caco2 cells showed that 80% exhibited abnormal actin fibre morphology, compared with SIPA1L3+GFP (P < 0.05). This result is consistent with p.Asp148Tyr affecting the predicted actin binding of SIPA1L3 (19). Caco2 cells transfected with D148Y+GFP exhibited fragmentation and reduced intensity of apical E-cadherin at cell–cell contacts (Fig. 7N–P). These findings suggest that the p.Asp148Tyr missense variant of SIPA1L3 affects actin stress fibre structure, as well as adhesion between epithelial cells. Discussion The identity and functional roles of many of the factors required for proper epithelial cell function and lens morphogenesis are not yet fully known. Through the study of a balanced 5796 | Human Molecular Genetics, 2015, Vol. 24, No. 20 Figure 5. Reduced expression of Sipa1l3 in mouse leads to abnormal lens development, cataract and microphthalmia. (A) Placement of the gene trap (GT) vector inserted into intron 3 of Sipa1l3 (EUCOMM). The gene trap consisted of a FlipRosaβgeo vector (35) with lacZ reporter. Germline transmission in chimeric mice was confirmed after the F1 generation by PCR. (B) Sipa1l3 expression revealed by lacZ expression in the E14.5, E18.5, P10 and adult mouse lens. E, embryonic age; P, postnatal age. (C) qPCR analysis of E18.5 lens tissue showed a >95% reduction in Sipa1l3 transcript in Sipa1l3 −/− embryos, compared with WT. (D–I) Slit-lamp examination of age-matched mice of 129/SvJ background and C57Bl/6 background. (D) 129/SvJ WT Sipa1l3 +/+ eye showed no opacities in the cornea or lens, whereas (E) Sipa1l3 −/− eye exhibits a central, dense cataract at 4 weeks postnatal age. (F) C57Bl/6 WT Sipa1l3 +/+ eye shows no opacities in the cornea or lens, whereas (G) Sipa1l3−/− eye exhibits a central, dense cataract at 4 weeks postnatal age with lenticular-iridial adhesion. (H and I) Sipa1l3 −/− lens showed a large central opacity and is reduced in size. (J and K) Abnormalities in the histology of Sipa1l3 −/− lenses compared with Sipa1l3 +/+ lenses (haematoxylin and eosin stained). Large vacuoles and aggregates (arrowheads) formed in the Sipa1l3 −/− lens. Scale bar, 150 µm. Table 1. Genotype distribution of Sipa1l3 mice on R1-129 and C57Bl/6 backgrounds Sipa1l3+/+ No. (%) Sipa1l3 R1-129 7 (22) Sipa1l3 C57Bl/6 9 (21.4) Sipa1l3+/− No. (%) 18 (56) 22 (52.4) Sipa1l3−/− No. (%) Table 2. Cataract distribution in Sipa1l3 mice Total number of mice examined by slit lamp ( percentage exhibiting cataracts/percentage exhibiting cataracts and lenticular-iridial adhesions) Sipa1l3+/+ Sipa1l3+/− Sipa1l3−/− 7 (22) 11 (26.2) chromosomal translocation patient, we identified SIPA1L3 as a novel candidate gene with a critical function in lens and eye development. Based on its predicted domain structure, SIPA1L3 is a member of the SIPA1 and SIPA1L families that possess RapGAP and PDZ domains. The Rap1 signalling cascade is implicated in the regulation of cell adhesion, polarity and cytoskeletal organization (13). We undertook cell-based, zebrafish and mouse model studies to investigate the functional role of SIPA1L3. Sipa1l3 R1-129 5 (0) Sipa1l3 C57Bl/6 13 (0) 13 (0) 12 (100) 23 (0) 14 (100/30) SIPA1L3 knockdown in a 3D cyst assay demonstrated abnormal cell polarity and morphogenesis. Disruption of Sipa1l3 function in zebrafish and mouse revealed lens and eye abnormalities. In the lens of the mutant mouse line, there were abnormalities of cell polarity, adhesion and cytoskeletal structure, and abnormal EMT. We also identified a missense variant of SIPA1L3 associated Human Molecular Genetics, 2015, Vol. 24, No. 20 | 5797 Figure 6. Epithelial lens cells of Sipa1l3−/− mouse show abnormal cellular adhesion, polarity and presence of EMT. (A) WT Sipa1l3 +/+ E18.5 mouse embryo shows normal lens and eye morphology. (B) The lens of Sipa1l3 −/− embryo shows no distinct lens fibres and germinative region (*), and a vacuolated region between the anterior epithelium and posterior lens fibres (arrowhead). (C) Magnified image of the anterior epithelium in Sipa1l3 +/+ E18.5 embryo shows an organized monolayer of columnar epithelial cells. (D) Sipa1l3 −/− eye shows a flattened layer of anterior epithelial cells with double layers (with overlapping nuclei, arrows). (E) Magnified image of the germinative/transition zone of the lens in Sipa1l3 +/+ E18.5 embryo shows typical anterior epithelial cells differentiating into lens fibre cells. (F) Lens of Sipa1l3 −/− embryo shows clumping of anterior epithelial cells (arrow) with no distinct elongated lens fibre cells in the transition zone. (G) Anterior lens epithelium of Sipa1l3 +/+ E18.5 embryo with basal and lateral E-cadherin staining, and an organized monolayer of anterior lens epithelial cells. F-actin marks the apical surface of the anterior epithelial cell layer. In contrast, the anterior lens epithelium of (H) Sipa1l3 −/− embryos shows disorganized arrangement of cell layers (arrow). Asterisk indicates individual nuclei. Scale bar, 10 µm. (I) The anterior epithelial cell region shows organized arrangement of the anterior monolayer. (J) The anterior epithelium of Sipa1l3 −/− embryo shows disrupted ZO-1 and F-actin continuity (arrow) and disorganized arrangement of cells (arrowhead). Scale bar, 10 µm. (K) The germinative region in Sipa1l3 +/+ embryo shows typical Factin and ZO-1 staining (arrow) and the normal transition into the fibre cell layer. (L) Sipa1l3 −/− embryo lens shows loss of ZO-1 and F-actin in the apical region of the anterior epithelial and fibre cell layer (arrow) and undulation of lens fibre cells (arrowhead). Scale bar, 50 µm. (M) The anterior lens epithelium of Sipa1l3 +/+ E18.5 embryo shows no αSMA (red) expression. (N) Sipa1l3 −/− embryos show expression of αSMA in the anterior lens epithelial cells (arrows). Arrowhead indicates multilayered cells. Scale bar, 10 µm. (O) Sipa1l3 +/+ E18.5 embryo lens shows no αSMA expression. (P) Sipa1l3 −/− embryo shows expression of αSMA in the anterior (arrows) and germinative zone (arrowhead). Asterisk indicates positive staining in the iris/ciliary body tissue in both WT and mutant embryos, as expected. Scale bar, 50 µm. 5798 | Human Molecular Genetics, 2015, Vol. 24, No. 20 Figure 7. Mutation of SIPA1L3 in epithelial cells impacts on cytoskeletal organization and cell adhesion. (A) The location of mutation p.Asp148Tyr ( p. D148Y) in the conserved region in the predicted actin-binding domain of SIPA1L3 in Patient 2. Other predicted functional domains are shown. (B–M) Colocalization of SIPA1L3 with basal F-actin stress fibres in Caco2 cells in 2D culture and in cells expressing the p.Asp148Tyr (D148Y)-mutant allele (E, I and M are enlargements of the boxed regions in D, H and L, respectively). (B–E) Basal region of the Caco2 cells transfected with control GFP (green) and stained for F-actin (red). Arrows indicate the presence of normal basal stress actin fibres in (C), (D and E) [enlargement of boxed region in (D)]. (F–I) Basal region of the Caco2 cells transfected with SIPA1L3+GFP (green) and stained for F-actin (red). Arrowhead in (F) indicates staining of SIPA1L3+GFP. Arrows indicate the presence of normal basal stress actin fibres in (G) and colocalization with SIPA1L3 in (H) and (I) [enlargement of boxed region in (H)]. (J–M) Basal region of the Caco2 cells transfected with D148Y+GFP and stained for F-actin. The D148Y +GFP showed a clustered distribution, arrowhead in (J), rather than the typical actin fibres observed with normal SIPA1L3+GFP, arrowhead in (F). The F-actin in the D148Y+GFP-transfected cells lost the stress actin fibre appearance (arrowhead in K), compared with untransfected cells (arrows in D) or cells transfected with SIPA1L3 +GFP (arrow in G) or GFP only (arrow in C). (L) Merged image shows the clustered appearance of the F-actin and D148Y+GFP in D148Y+GFP-transfected cells (arrowhead with enlarged region shown in (M). (N and O) Caco2 cells transfected with control GFP, SIPA1L3+GFP and D148Y+GFP, and stained for E-cadherin in the apical region. Cells transfected with D148Y+GFP showed fragmentation of E-cadherin staining compared with GFP alone or SIPA1L3+GFP. (P) E-cadherin fluorescence intensity measured in Caco2 cells transfected with control GFP, SIPA1L3+GFP and D148Y+GFP. D148Y+GFP-transfected cells show a significant reduction in fluorescence intensity, compared with control GFP, whereas SIPA1L3+GFP-transfected cells exhibited no significant difference in intensity compared with control GFPtransfected cells (n = 28 per construct, ***P < 0.001 by ANOVA). Fluorescence intensity analysis was performed using ImageJ Software. Human Molecular Genetics, 2015, Vol. 24, No. 20 with abnormal cellular actin fibre formation. Our findings demonstrate that Sipa1l3 is critical in epithelial cell morphogenesis, polarity, cytoskeletal structure, adhesion and prevention of EMT in the lens. Many of the underlying factors and their roles in regulating epithelial cell morphogenesis and EMT are still unknown. This study has used interrogation of biological findings in human patients to reveal a novel factor required for proper epithelial cell formation. The loss of polarity and abnormal morphology observed in our SIPA1L3 knockdown cyst cultures, as well as in the lens epithelial cells of the Sipa1l3 −/− mutant mice, suggested a failure in the maintenance of epithelial cell properties. Mice deficient in the polarity protein, aPKCλ, which develop cataracts, show abnormal organization of fibre and anterior epithelial cells, as well as induction of EMT in the anterior lens epithelial cells (3). This is similar to findings in our mutant Sipa1l3 −/− mice, suggesting that both fibre cell disorganization and anterior epithelial cell abnormalities may contribute to the cataract formation. Failure in the maintenance of epithelial cell properties can result in abnormal EMT, characterized by the disassembly of cell adhesion, loss of polarity and the acquisition of migratory capacity (41). Induction of EMT has also been demonstrated to contribute to cataracts and lens defects in mutant mice with lens-specific deletion of a PDZ-containing protein called Scribble (42). ScribLeCre mice exhibited small and vacuolated lens that lost E-cadherin and ZO-1 proteins, while concomitantly showing an increase in αSMA (43). Lens-specific activation of TGFβ in mice has also been shown to induce EMT and the formation of anterior subcapsular cataracts (44). In our study, lens epithelial cells of Sipa1l3-mutant mice exhibited loss of proper cytoskeletal organization, adhesion and polarity and showed aberrant expression of αSMA, indicating a role for Sipa1l3 in the regulation of EMT. SIPA1L1 harbours two regions of low homology found to associate with actin (19,20), but nothing was previously known about the function of this region in SIPA1L3. Our second patient (Patient 2) had a missense variant in SIPA1L3, p.Asp148Tyr, in a region homologous to the first putative actin-binding domain of SIPA1L1. Our study showed that the presence of this variant led to a lack of normal basal actin stress fibre formation and absence of normal SIPA1L3 and F-actin colocalization, showing this region to be an actin-binding domain in SIPA1L3. Loss of expression of Sipa1l3 caused lens and eye anterior segment abnormalities in our Sipa1l3 homozygous loss-of-function mutant mice. Patient 1 in this study carried a balanced chromosomal translocation transecting the 5′ UTR of SIPA1L3 associated with reduced expression of SIPA1L3 in lymphoblasts. While heterozygous loss of function was not associated with an eye phenotype in our mutant mouse line, there is variability in gene dosage requirements between species (45), so this does not exclude the possibility that haploinsufficiency may be causing or contributing to the eye phenotype in Patient 1. There are other examples in lens and development eye disease where heterozygous loss of function alleles do not have a phenotypic impact in animal models, but do in the human (46–48). Patient 2 in this study had a heterozygous missense variant in SIPA1L3, and our cell-based studies indicated abnormal function owing to this missense variant. While this manuscript was in submission, a homozygous nonsense variant in SIPA1L3 (c.4489C>T, p.R1497X) was identified in two siblings with congenital cataracts (24). This variant led to truncation of the conserved C-terminal coiled-coiled region of the protein, highlighting further considerations in the roles of the functional domains of SIPA1L3. It is noteworthy that a variety of mutational mechanisms such as loss and altered function in | 5799 other genes, including GJA8/Gja8 (10,49), MAB21L2/mab21l2 (46,50) and FOXE3/Foxe3 (51–53), may lead to lens and developmental eye anomalies in humans and mice. While the translocation abnormality and reduced expression of SIPA1L3 in Patient 1 led us to consider SIPA1L3 as a candidate disease gene, and the missense variant in Patient 2 caused altered function of SIPA1L3 in our cell-based assay, it is also possible that variation in another gene or genes may be responsible for, or contributing to, the phenotype of both these patients. Examination of further patients is required to determine the SIPA1L3 mutational spectrum capable of leading to eye abnormalities. Our work highlights the complex mechanisms and network of signalling, transcription and downstream factors required for proper lens and eye development that continue to be unravelled. In our study, we noted that disruption of SIPA1L3 function in the colorectal adenocarcinoma cell line, Caco2, led to abnormalities of epithelial cell morphogenesis, cytoskeletal structure and adhesion. Such abnormalities are also frequently found in cancer cells. Indeed, interrogation of the cBioPortal for Cancer Genomics revealed that somatic abnormalities of SIPA1L3 are present in over 40 cancer types, many of epithelial origin (54) including in Asp148, suggesting that dysfunction of SIPA1L3 might also contribute to the phenotype of some malignancies. Our results point to a role for SIPA1L3 in normal epithelial cell behaviour and the regulation of EMT. In addition to its role in epithelial cell morphogenesis, maintenance of normal SIPA1L3 level and function may be a critical factor in the prevention of invasive properties of some somatic epithelial cell cancers. This is likely to be a context or tissue-specific effect, as none of our patients or knockout mice are known to have malignancy. We found in this study that dysfunction of SIPA1L3 led to lens and eye abnormalities in human, zebrafish and mouse. Reduced expression in mouse, and cell line studies indicated that Sipa1l3 is required for correct epithelial cell morphogenesis, polarity and cytoskeletal organization. This study identifies SIPA1L3 as a new factor for the investigation of pathogenic mechanisms underlying epithelial cell abnormalities and the control of normal epithelial cell behaviour. Materials and Methods Patient recruitment Patients included in this study were recruited through joint ophthalmic genetics clinics at The Children’s Hospital at Westmead, Sydney, Australia, St Mary’s Hospital, Manchester, UK and a research protocol of the Children’s Hospital of Wisconsin, USA. Written informed consent was obtained for each affected individual. This research followed the tenets of the Declaration of Helsinki, and ethics committee approval was obtained from The Children’s Hospital at Westmead, Sydney, Australia, the North West Research Ethics Committee, Manchester, UK and the Institutional Review Board at the Children’s Hospital of Wisconsin, USA. Patients had tested negative for mutations in known genes associated with congenital cataracts and other developmental eye abnormalities (4,55). Genomic analyses and next-generation sequencing Chromosome microarray (CMA) testing in Patient 1 was performed using Agilent’s SurePrint G3 Targeted Chromosome Microarray (8 × 60K) following the manufacturer’s protocol (Agilent Technologies, Santa Clara CA, USA). Commercial male control DNA (Promega, Madison, WI, USA) was used to achieve 5800 | Human Molecular Genetics, 2015, Vol. 24, No. 20 comparative hybridization. Agilent’s Genomic Workbench Standard Edition Version 5.0.14 Quality Control data metrics were assessed prior to data interpretation (DLR < 0.25, signal-tonoise ratio >20 units). Final probe data were analysed at an effective mean resolution of >0.2 Mb, where duplication or deletion was defined as log2 ≥0.5 or ≤−1.0, respectively. Next-generation sequencing in Patient 1 was performed using the Illumina Trusight Clinical Exome (Illumina, San Diego, CA, USA) for capture, followed by sequencing on the HiSeq2000 (Illumina; Ramaciotti Sequencing Centre, University of NSW), and known ASD/cataract/microphthalmia genes were analysed using our bioinformatics pipeline (4). A separate targeted amplicon approach was used to cover SIPA1L3, using the Truseq Custom Amplicon (Illumina) and sequenced on the Illumina Miseq (Illumina) with 2 × 250 bp paired-end reads (Ramaciotti Centre, Sydney, Australia). Gaps with coverage of <5× were analysed with conventional bidirectional Sanger sequencing. NGS in Patient 2 was performed using whole exome capture on the Agilent Sure Select v4 platform and sequencing on the Illumina HiSeq 2000 system, with bioinformatic analysis as described in Reis et al. (56). Breakpoint mapping in Patient 1 was performed using somatic cell hybrids containing only derivative chromosomes 2 or 19, as described previously (8). Sequencetagged site markers were selected according to initial breakpoint location approximated from karyotype analyses, with flanking primer sequences shown in (Supplementary Material, Table S1). Long-range PCR (Roche, Australia) was utilized to amplify the sequence across the breakpoints on derivative chromosomes 2 and 19 (Supplementary Material, Table S1). NGS of SIPA1L3 in 80 other patients with lens, other eye anterior segment abnormalities or microphthalmia was performed using either a targeted amplicon approach using the Truseq Custom Amplicon (Illumina), or an NGS strategy using long-range PCR fragments using the NexteraXT DNA Sample Preparation kit (Illumina). Index-tagged library amplicons were pooled together prior to on-board cluster generation and massively parallel sequencing on the MiSeq platform (Illumina) according to the manufacturer’s instructions. Primer design Primers were designed as required for SIPA1L3 according to NM_015073 using Primer3 (version 0.4.0) software (http://frodo. wi.mit.edu/primer3/) for the analysis of coding exons and immediately flanking intronic sequences. Primers for long-range PCR (LR-PCR) were designed to generate amplicons ∼8.5 Kb in size. Bioinformatics, in silico analysis and variant prioritization Sequenced reads from the MiSeq or HiSeq were aligned to the human reference sequence (hg19) with Stampy (v1.0.17) or BWA software. The genome analysis tool kit Lite (GATK v2.0.39) was used for base quality score recalibration and indel realignment prior to variant calling using the unified Genotyper. Singlenucleotide polymorphisms (SNPs) with ≥5× coverage and indels were annotated to genes using Ensembl v68, and the functional consequences were then defined against the SIPA1L3 RefSeq transcript, NM_015073, and known disease genes where required (4). Additional annotation was provided from OMIM and Genomic Evolutionary Rate Profiling (35 species alignment) as well as population frequencies from the 1000 genomes project ( phase 1 release) and the NHLBI Exome Sequencing Project (v6500). PolyPhen-2 and SIFT predictions were also included to help determine pathogenicity. PCR and Sanger sequencing PCR amplicons used for Sanger sequencing were purified using AMPure (Beckmann Coulter, Brea, CA, USA) or column purification using the Wizard® SV Gel and PCR Clean-up System (Promega, Australia) according to manufacturer instructions. Sequencing was conducted using BigDye Terminator v3.1 Cycle Sequencing Kit and ABI3730XL sequencing (Applied Biosystems, Life Technologies, Carlsbad, CA, USA) according to manufacturer instructions or outsourced to Macrogen, Inc. (Seoul, Korea). Cell culture and cyst production Caco2 and MDCK cells were maintained in Dulbecco’s modified Eagle’s medium (GIBCO) with 10% fetal calf serum (Life Technologies, Australia) at 37°C in 5% CO2. shRNA Caco2 cell lines were created by lentiviral-mediated transduction using calcium phosphate and shRNA vectors custom ordered from Sigma–Aldrich, USA. Caco2 cells were transiently transfected with a SIPA1L3 +GFP construct purchased from Origene, USA (Product number RG220237). D148Y+GFP construct was created using SIPA1L3 +GFP and In-Fusion® HD Cloning System (Clontech, USA) according to manufacturer’s instructions. Transient transfections were performed using Lipofectamine 2000 (Life Technologies, Australia) according to manufacturer instructions. Caco2 cells were used to create cyst structures using a three-dimensional culture system as previously described (34). In brief, subconfluent Caco2 cells were trypsinized and added to a collagen solution (24 m GlutaMAX, 2.8 m NaHCO3, 1× MEM, 20 m HEPES, 2 mg/ml collagen I) to a final concentration of 3 × 104 cells/ml. A concentration of 150 µl of collagen/cell mixture was placed into the centre of a 12-mm Transwell® polycarbonate membrane insert (Corning, USA) and incubated for 45 min at 37°C in 0% CO2. Culture medium was added to the wells, and the cells were maintained at 37°C in 5% CO2. Cell immunofluorescence Caco2 and MDCK cells in two-dimensional cultures were incubated with mouse monoclonal anti-E-cadherin (Life Technologies), rabbit polyclonal anti-PKC ζ (aPKC) (Santa Cruz Biotechnology, USA) and Alexa-594-conjugated phalloidin (Life Technologies). Cells were also incubated with Alexa-488 goat anti-mouse secondary, Alexa-488 goat anti-rabbit secondary antibody (Life Technologies) and DAPI (Sigma–Aldrich, Australia) and imaged by a Leica SP5 II Confocal microscope (Leica Microsystems, Australia). Immunofluorescence of Caco2 cells in three-dimensional cultures was undertaken as previously described (34). In brief, Transwell® inserts were washed with PBS+ and treated with 100 U/ml collagenase-4 at 37°C for 10 min, followed by three quick washes. Cells were fixed in 4% PFA for 30 min with slow shaking at room temperature. Transwell® inserts were washed with PBS+ (three quick, three 10 min) and blocked with permeablization solution (10% FCS, 0.5% Triton X-100 in PBS+) for 30 min at room temperature. Primary antibodies were diluted in permeablization solution and incubated overnight at 4°C. Transwell® inserts were washed with PBS+ (three quick, three 10 min), and secondary antibodies coupled with Alexa-488 fluorophores and DAPI were added to the inserts and incubated for 2 h with shaking at room temperature. Cells were mounted using DABCO® (Sigma–Aldrich, Australia) and imaged by a Leica SP5 II Confocal microscope (Leica Microsystems). Fluorescence intensity analysis was performed using ImageJ Software (57). Human Molecular Genetics, 2015, Vol. 24, No. 20 RNA isolation, cDNA synthesis and quantitative real-time PCR Mouse and zebrafish tissue, and cells were collected and processed according to manufacturer’s instructions using RNeasy® Lipid Tissue Mini Kit (Qiagen, Australia) for RNA isolation. cDNA was synthesized using SuperScript® III Reverse Transcriptase (Life Technologies, Australia), according to the manufacturer instructions. RT-PCR analysis for mouse Sipa1l3 expression was performed using two primers: 5′ GACCTCAGTACCAGTACAG 3′ and 5′ GCTGCCTGTTGTTGGGAGTG 3′; and for zebrafish Sipa1l3 expression: 5′ GAGGTGGTGTTTAACTGTTTCTG 3′ and 5′ GATTT CAACCAATCGGCTTCCCTG 3′. qPCR analysis for human SIPA1L3 expression in cell lines was performed using two primers: 5′ CACGATGTACCAGGACTAC 3′ and 5′ CAGGCTCCTGGAA GATGATC 3′. qPCR was performed using Immolase™ DNA polymerase (Bioline, Australia) with an annealing temperature of 58°C for 32 cycles using Rotor-Gene 6000 (Corbett Life Science, Australia) and analysed by standard curve method using RotorGene 6000 software(58). We performed quantitative PCR for human SIPA1L3 expression in Patient 1 using FAM-labelled commercial assay probes. RT-PCR was performed using first-strand cDNA with TaqMan Fast Universal PCR Master Mix (Applied Biosystems, USA). The assay numbers for the target and mRNA endogenous control (GAPDH) genes were as follows: GAPDH (Hs02758991_g1) and SIPA1L3 (Hs00390580_m1) (all from Life Technologies, USA). Quantitative PCR was then performed on the Applied Biosystems Real-Time PCR system (7900 HT Fast Real-Time PCR machine, Applied Biosystems). Reaction conditions used were as follows: 95°C for 10 min; then 40 cycles of PCR at 95°C for 15 s and 60°C for 1 min. Reactions were performed in triplicates in 10 μl reaction volume. Cycles to threshold (CT) values were then determined for each sample and its matched control and relative mRNA expression levels determined using the 2−ΔΔCt method (59), providing the fold change (RQ) value. Zebrafish Zebrafish experiments were approved by the Animal Ethics Committee of the University of Sydney and performed at the Brain and Mind Research Institute, University of Sydney. Casper zebrafish (60) were bred and maintained under standard conditions on a 14 h light/10 h dark cycle. Embryos were collected by natural spawning, raised at 28.5°C and staged by hours post-fertilization (hpf ) or days post fertilization (dpf )(61). Zebrafish in situ, immunofluorescence and histology Whole-mount in situ hybridization was performed using a riboprobe for sipa1l3. A 528-bp cDNA fragment was amplified using two primers: 5′ GATCGTCGACGCAAGATATCAGAGAGGTGC 3′ (Forward) and 5′ GATCGCGGCCGCGTTGCGGCTCTCTGTATGG 3′ (Reverse). Riboprobe synthesis was performed using the Roche-DIG RNA Labeling Kit (SP6/T7) (Roche Applied Science, Australia). Zebrafish embryos were fixed and hybridized with the sipa1l3 riboprobe and imaged using the Leica M2-16 microscope (Leica Microsystems). Whole-mount immunofluorescence staining was performed on 24 hpf and 72 hpf zebrafish embryos using rabbit polyclonal anti-sipa1l3 (Abcam, USA) and Alexa-594 Donkey anti-rabbit antibody (Life Technologies, Australia). Immunofluorescence staining was captured by SteREO Lumar.V12 Zeiss microscope (Carl Zeiss Pty Ltd, Australia). Zebrafish were embedded in Poly/Bed®812 solution (PolySciences, Inc., Germany) and sectioned semi-thin (0.5–1.0 µm). Slides were stained with 0.5% Toluidine Blue and mounted with DPX mounting medium | 5801 (Sigma–Aldrich, Australia). Slides were imaged by Axio Imager A1 Zeiss microscope (Carl Zeiss Pty Ltd). Zebrafish morpholinos Antisense morpholino oligonucleotides for sipa1l3 were designed and purchased from GeneTool, LLC, along with a standard control morpholino (5′ CCTCTTACCTCAGTTACAATTTATA 3′). Two morpholinos were used: MO1 (5′ TATTTCCCTGGCATACACACC TCGC 3′), targeted at the exon 2/intron2 splice junction, and MO2 (5′ GAGACAGAAACTCATGCTCACTTTT 3′), targeted at the exon 4/intron 4 splice junction. Morpholino (8 ng/µl) volumes were calibrated to 1.77–2 nl and injected into one-cell-stage zebrafish embryos. Embryos were incubated at 28.5°C and imaged at 5 dpf using Lumar.V12 Zeiss microscope (Carl Zeiss Pty Ltd). Approximately 200 embryos were injected independently with sipal13 morpholinos (MO1 or MO2) and control morpholino. Expression analysis of results of sipa1l3 morpholinos used in zebrafish was performed using the following primers: Exon 1 Forward 5′ GAACGTAACGTCAGCTTTTTGAG 3′ with Exon 3 Reverse 5′ GTACTCCAGAACCTCCTTTAAAG 3′ for MO1; and Exon 3 Forward 5′ CTTTAAAGGAGGTTCTGGAGTAC 3′ with Exon 5 Reverse 5′ CATGTATGGAAGCATGGTGGACAC 3′ for MO2. β-actin primers were used as control (Forward 5′ CGGAATATCATCTGCTTGTAAC 3′ and Reverse 5′ CGACCCACGATGGATGGGAAGAC 3′). Mouse mutant Mouse experiments were approved by the Animal Care and Ethics Committee of the Children’s Medical Research Institute, Sydney, Australia. Sipa1l3-mutant mouse embryonic stem cells were purchased from the European Conditional Mouse Mutagenesis Program (EUCOMM, Germany), which contained a gene trap vector, FlipRosaβgeo (35), positioned in intron 3 of Sipa1l3. Transgenic mice were maintained on a 129/SvJ background and a C57BL/6 background. Slit-lamp (Leica Microsystems) analysis was performed at 4 weeks postnatal age. PCR genotyping was performed using two sets of primers: one amplifying the WT Sipa1l3 allele (5′ GTCTGTCTGCCAGGTACTTCCCCTG 3′ and 5′ GCGGCATCAAGC TATTTCCCTG 3′) and the other amplifying the gene trap allele (5′ GTCTGTCTGCCAGGTACTTCCCCTG 3′ and 5′ GCAGATCCAT ACCCTGCTTG 3′). Mouse histology, immunohistochemistry and lacZ staining Embryos, whole heads and eyes were embedded in paraffin and sectioned at 5 μm for histology. Sections were stained with haematoxylin and eosin, mounted with Ultramount (Fronine, Australia) and imaged by Axio Imager A1 Zeiss microscope (Carl Zeiss Pty Ltd). Embryos, whole heads and eyes were embedded in 30% sucrose and Optimal Cutting Temperature compound (TissueTek®, Australia) and sectioned at 5 μm for immunohistochemistry and lacZ staining. Immunohistochemistry was performed using mouse monoclonal anti-E-cadherin (Life Technologies, USA), Alexa-488-conjugated anti-ZO-1 (#13-1900 Invitrogen, Australia), anti-αSMA (Sigma–Aldrich, Australia) and Alexa-594-conjugated phalloidin (Life Technologies, USA). Slides were incubated in Alexa-488 goat anti-mouse secondary antibody (Life Technologies) and DAPI (Sigma–Aldrich, Australia) and then mounted in 70% glycerol. Slides were imaged by a Leica SP5 II Confocal microscope (Leica Microsystems). Sections for lacZ staining were incubated with X-gal (Sigma–Aldrich, Australia) and counterstained with nuclear fast red (Sigma–Aldrich, 5802 | Human Molecular Genetics, 2015, Vol. 24, No. 20 Australia), mounted with Ultramount (Fronine) and imaged using an Axio Imager A1 (Zeiss, Australia). 8. Supplementary Material Supplementary Material is available at HMG online. Acknowledgements We thank the patients for their involvement in this research. We acknowledge Martin Carette for the generation of derivative chromosomal cell lines, the EUCOMM Consortium for the provision of gene trap mouse embryonic stem cell clones and Tania Radziewic for microinjections required to create the mutant mice. We acknowledge use of the Westmead Research Hub’s Leica TCS SP5 at the Kid’s Research Institute for confocal imaging, and Laurence Cantrill for his assistance. We acknowledge the support from the Raymer family. Confocal and optical microscopy at IMB was performed at the ACRF/IMB Cancer Biology Imaging Facility, established with the support of the Australian Cancer Research Foundation. We acknowledge support of the Manchester Academic Health Science Centre and the Manchester National Institute for Health Research Biomedical Research Centre. 9. 10. 11. 12. 13. Conflict of Interest statement. None declared. 14. Funding 15. This work was supported by the National Health and Medical Research Council of Australia (Grant 1008194 to R.V.J.; Grants 1037320, 1067405, 1044041 to A.Y.; Grant 1003100 to P.P.L.T.), Ophthalmic Research Institute of Australia (to R.V.J. and J.R.G.), Cure Cancer Australia and Tour de Cure (to D.S.). References 1. Wormstone, I. and Wride, M. (2011) The ocular lens: a classic model for development, physiology and disease. Philos. Trans. R. Soc. Lond. B Biol. Sci., 366, 1190–1192. 2. Pontoriero, G., Smith, A., Miller, L., Radice, G., West-Mays, J. and Lang, R. (2009) Co-operative roles for E-cadherin and Ncadherin during lens vesicle separation and lens epithelial cell survival. Develop. Biol., 326, 403–417. 3. Sugiyama, Y., Akimoto, K., Robinson, M., Ohno, S. and Quinlan, R. (2009) A cell polarity protein aPKClambda is required for eye lens formation and growth. Dev. Biol., 336, 246–256. 4. Prokudin, I., Simons, C., Grigg, J., Storen, R., Kumar, V., Phua, Z., Smith, J., Flaherty, M., Davila, S. and Jamieson, R. (2013) Exome sequencing in developmental eye disease leads to identification of causal variants in GJA8, CRYGC, PAX6 and CYP1B1. Eur. J. Hum. Genet., 22, 907–915. 5. Hanson, I., Fletcher, J., Jordan, T., Brown, A., Taylor, D., Adams, R., Punnett, H. and van Heyningen, V. (1994) Mutations at the PAX6 locus are found in heterogeneous anterior segment malformations including Peters’ anomaly. Nat. Genet., 6, 168–173. 6. Semina, E., Ferrell, R., Mintz-Hittner, H., Bitoun, P., Alward, W., Reiter, R., Funkhauser, C., Daack-Hirsch, S. and Murray, J. (1998) A novel homeobox gene PITX3 is mutated in families with autosomal-dominant cataracts and ASMD. Nat. Genet., 19, 167–170. 7. Semina, E., Brownell, I., Mintz-Hittner, H., Murray, J. and Jamrich, M. (2001) Mutations in the human forkhead 16. 17. 18. 19. 20. 21. 22. 23. 24. transcription factor FOXE3 associated with anterior segment ocular dysgenesis and cataracts. Hum. Mol. Genet., 10, 231–236. Jamieson, R., Perveen, R., Kerr, B., Carette, M., Yardley, J., Heon, E., Wirth, M., van Heyningen, V., Donnai, D., Munier, F. and Black, G. (2002) Domain disruption and mutation of the bZIP transcription factor, MAF, associated with cataract, ocular anterior segment dysgenesis and coloboma. Hum. Mol. Genet., 11, 33–42. Medina-Martinez, O., Shah, R. and Jamrich, M. (2009) Pitx3 controls multiple aspects of lens development. Dev. Dynam., 238, 2193–2201. Chang, B., Wang, X., Hawes, N.L., Ojakian, R., Davisson, M.T., Lo, W.K. and Gong, X. (2002) A GJA8 (Cx50) point mutation causes an alteration of alpha 3 Connexin (Cx46) in semi-dominant cataracts of Lop10 mice. Hum. Mol. Genet., 11, 507–513. Beby, F., Commeaux, C., Bozon, M., Denis, P., Edery, P. and Morle, L. (2007) New phenotype associated with an Arg116Cys mutation in the CRYAA gene: nuclear cataract, iris Coloboma, and Microphthalmia. Arch. Ophthalmol. Chic., 125, 213–216. Maddala, R., Chauhan, B., Walker, C., Zheng, Y., Robinson, M., Lang, R. and Rao, P. (2011) Rac1 GTPase-deficient mouse lens exhibits defects in shape, suture formation, fibre cell migration and survival. Dev. Biol., 360, 30–43. Kooistra, M., Dube, N. and Bos, J. (2007) Rap1: a key regulator in cell-cell junction formation. J. Cell Sci., 120, 17–22. Hattori, M. and Minato, N. (2003) Rap1 GTPase: functions, regulation, and malignancy. J. Biol. Chem., 134, 479–484. Pannekoek, W., Kooistra, M., Zwartkruis, F. and Bos, J. (2009) Cell-cell junction formation: the role of Rap1 and Rap1 guanine nucleotide exchange factors. Biochim. Biophys. Acta, 1788, 790–796. Hattori, M., Tsukamoto, N., Nur-e-Kamal, M., Rubinfeld, B., Iwai, K., Kubota, H., Maruta, H. and Minato, N. (1995) Molecular cloning of a novel mitogen-inducible nuclear protein with a ran GTPase-activating domain that affects cell cycle progression. Mol. Cell. Biol., 15, 552–560. McAvoy, T., Zhou, M., Greengard, P. and Nairn, A. (2009) Phosphorylation of Rap1GAP, a striatally enriched protein, by protein kinase A controls Rap1 activity and dendritic spine morphology. Proc. Natl Acad. Sci. USA, 106, 3531–3536. Wienecke, R., Konig, A. and DeClue, J. (1995) Identification of tuberin, the tuberous sclerosis-2 product. tuberin possesses specific Rap1GAP activity. J. Biol. Chem., 270, 16409–16414. Pak, D., Yang, S., Rudolph-Correia, S., Kim, E. and Sheng, M. (2001) Regulation of dendritic spine morphology by SPAR, a PSD-95-associated RapGAP. Neuron, 31, 289–303. Tsai, I., Amack, J., Gao, Z., Band, V., Yost, H. and Virshup, D. (2007) A Wnt-CKIvarepsilon-Rap1 pathway regulates gastrulation by modulating SIPA1L1, a Rap GTPase activating protein. Dev. Cell, 12, 335–347. Matsuda, M., Kobayashi, Y., Masuda, S., Adachi, M., Wantanabe, T., Yamashita, J., Nishi, E., Tsukita, S. and Furuse, M. (2008) Identification of adherens junction-associated GTPase activating proteins by the fluorescence localization-based expression cloning. Exp. Cell Res., 314, 939–949. Spilker, C., Acuna Sanhueza, G., Bockers, T., Kreutz, M. and Gundelfinger, E. (2008) SPAR2, a novel SPAR-related protein with GAP activity for Rap1 and Rap2. J. Neurochem., 104, 187–201. Spilker, C. and Kreutz, M. (2010) RapGAPs in brain: multipurpose players in neuronal rap signalling. Eur. J. Neurosci., 32, 1–9. Evers, C., Paramasivam, N., Hinderhofer, K., Fischer, C., Granzow, M., Schmidt-Bacher, A., Eils, R., Steinbeisser, H., Schlesner, M. and Moog, U. (2015) SIPA1L3 identified by Human Molecular Genetics, 2015, Vol. 24, No. 20 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. 36. 37. 38. 39. 40. linkage analysis and whole-exome sequencing as a novel gene for autosomal recessive congenital cataract. Eur. J. Hum. Genet., doi:10.1038/ejhg.2015.46. Lachke, S., Salil, A., Ho, J., Kryukov, G., O’Connell, D., Aboukhalil, A., Bulyk, M., Park, P. and Maas, R. (2012) iSyTE: integrated systems tool for eye gene discovery. Invest. Ophth. Vis. Sci., 53, 1617–1627. Bastian, F., Parmentier, G., Roux, J., Moretti, S., Laudet, V. and Robinson-Rechavi, M. (2008) In Bairoch, A., Cohen-Boulakia, S. and Froidevaux, C. (eds.), Data Integration in the Life Sciences. Springer, Berlin Heidelberg, pp. 124–131. Kurachi, H., Wada, Y., Tsukamoto, N., Maeda, M., Kubota, H., Hattori, M., Iwai, K. and Minato, N. (1997) Human SPA-1 gene product selectively expressed in lymphoid tissues is a specific GTPase-activating protein for Rap1 and Rap2. Segregate expression profiles from a rap1GAP gene product. J. Biochem., 272, 28081–28088. Buckland, P. (2004) Allele-specific gene expression differences in humans. Hum. Mol. Genet., 13, R255–R260. Suzuki, S., Nakao, A., Sarhat, A., Furuya, A., Matsuo, K., Tanahashu, Y., Kajino, H. and Azuma, H. (2014) A Case of Pancreatic Agenesis and Congenital Heart Defects with a Novel GATA6 Nonsense Mutation: Evidence of Happloinsufficiency due to Nonsense-Mediated mRNA Decay. Am. J. Hum. Genet., 164A, 476–479. Hogan, C., Serpente, N., Cogram, P., Hosking, C., Bialucha, C., Feller, S., Braga, V., Birchmeier, W. and Fujita, Y. (2004) Rap1 regulates the formation of E-cadherin-based cell-cell contacts. Mol. Cell. Biol., 24, 6690–6700. Reedquist, K., Ross, E., Koop, E., Wolthuis, R., Zwartkruis, F., van Kooyk, Y., Salmon, M., Buckley, C. and Bos, J. (2000) The small GTPase, Rap1, mediates CD31-induced integrin adhesion. J. Cell Biol., 148, 1151–1158. Wu, C., Ramirez, A., Cui, B., Ding, J., Celcroix, J., Valletta, J., Liu, J., Yang, Y., Chu, S. and Mobley, W. (2007) A functional dyeninmicrotubule network is required for NGF signaling through the Rap1/MAPK pathway. Traffic, 8, 1503–1520. Tsukamoto, N., Hattori, M., Yang, H., Bos, J. and Minato, N. (1999) Rap1 GTPase-activating protein SPA-1 negatively regulates cell adhesion. J. Biol. Chem., 274, 18463–18469. Elia, N. and Lippincott-Schwartz, J. (2009) Culturing MDCK cells in three dimensions for analyzing intracellular dynamics. Curr. Protoc. Cell Biol., Chapter 4, Unit 4.22. Schunutgen, F., De-Zolt, S., Van Sloun, P., Hollatz, M., Floss, T., Hansen, J., Altsmied, J., Seisenberger, C., Ghyselinck, N., Ruiz, P. et al. (2005) Genomewide production of multipurpose alleles for the functional analysis of the mouse genome. Proc. Natl Acad. Sci. USA, 102, 7221–7226. Hales, A., Chamberlain, C. and McAvoy, J. (1995) Cataract induction in lenses cultured with transforming growth factorbeta. Invest. Ophth. Vis. Sci., 36, 1709–1713. Ng, P. and Henikoff, S. (2003) SIFT: predicting amino acid changes that affect protein function. Nucl. Acid Res., 31, 3812–3814. Schwarz, J., Cooper, D., Schuelke, M. and Seelow, D. (2014) MutationTaster2: mutation prediction for the deep-sequencing age. Nat. Methods, 11, 361–362. Adzhubei, I., Schmidt, S., Peshkin, L., Ramensky, V., Gerasimova, A., Bork, P., Kondrashhov, A. and Sunyaev, S. (2010) A method and server for predicting damaging missense mutations. Nat. Methods, 7, 248–249. Exome Variant Server, NHLBI GO Exome Sequencing Project (ESP) (2014) http://evs.gs.washington.edu/EVS/ (date last accessed, 31 March 2014). | 5803 41. Kalluri, R. and Weinberg, R. (2009) The basics of epithelialmesenchymal transition. J. Clin. Invest., 119, 1420–1428. 42. Yamben, I., Rachel, R., Shatadal, S., Copeland, N., Jenkins, N., Warming, S. and Griep, A. (2013) Scrib is required for epithelial cell identity and prevents epithelial to mesenchymal transition in the mouse. Dev. Biol., 384, 41–52. 43. Yingling, J., Datto, M., Wong, C., Frederick, J., Liberati, N. and Wang, X. (1997) Tumor suppressor Smad4 is a transforming growth factor beta-inducible DNA binding protein. Mol. Cell. Biol., 17, 7019–7028. 44. Srinivasan, Y., Lovicu, F. and Overbeek, P. (1998) Lens-specific expression of transforming growth factor Beat1 in transgenic mice causes anterior subcapsular cataracts. J. Clin. Invest., 101, 625–634. 45. Zhang, Z. and Baldini, A. (2008) In vivo response to high-resolution variation of Tbx1 mRNA dosage. Hum. Mol. Genet., 17, 150–157. 46. Deml, B., Kariminejad, A., Borujerdi, R.H.R., Muheisen, S., Reis, L.M. and Semina, E.V. (2015) Mutations in MAB21L2 Result in Ocular Coloboma, Microcornea and Cataracts. PLoS Genet., 11, e1005002. 47. Pal, J., Liu, X., Mackay, D., Shiels, A., Berthoud, V., Beyer, E. and Ebihara, L. (2000) Connexin46 mutations linked to congenital cataract show loss of gap junction channel function. Am. J. Physiol. -Cell Ph., 279, C596–C602. 48. Gong, X., Li, E., Klier, G., Huang, Q., Wu, Y., Lei, H., Kumar, N., Horwitz, J. and Gilula, N. (1997) Disruption of alpha3 connexin gene leads to proteolysis and cataractogenesis in mice. Cell, 91, 833–843. 49. Ponnam, S.P.G., Ramesha, K., Matalia, J., Tejwani, S., Ramamurthy, B. and Kannabiran, C. (2013) Mutational screening of Indian families with hereditary congenital cataract. Mol. Vis., 19, 1141–1148. 50. Rainger, J., Pehlivan, D., Johansson, S., Bengani, H., SanchezPulido, L., Williamson, K.A., Ture, M., Barker, H., Rosendahl, K., Spranger, J. et al. (2014) Monoallelic and biallelic mutations in MAB21L2 cause a spectrum of major eye malformations. Am. J. Hum. Genet., 94, 915–923. 51. Bremond-Gignac, D., Bitoun, P., Reis, L., Copin, H., Murray, J. and Semina, E. (2010) Identification of dominant FOXE3 and PAX6 mutations in patients with congenital cataract and aniridia. Mol. Vis., 16, 1705–1711. 52. Iseri, S.U., Osborne, R.J., Farrall, M., Wyatt, A.W., Mirza, G., Nurnberg, G., Kluck, C., Herbert, H., Martin, A., Hussain, M. S. et al. (2009) Seeing clearly: the dominant and recessive nature of FOXE3 in eye developmental anomalies. Hum. Mutat., 30, 1378–1386. 53. Ormstad, M., Blixt, A., Churchill, A., Martinsson, T., Enerback, S. and Carlsson, P. (2002) Foxe3 haploinsufficiency in mice: a model for Peters’ anomaly. Invest. Ophth. Vis. Sci., 43, 1350– 1357. 54. Cerami, E., Gao, J., Dogrusoz, U., Gross, B., Sumer, S., Aksoy, B., Jacobsen, A., Byrne, C., Heuer, M., Larsson, E. et al. (2012) The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov., 2, 401–404. 55. Gillespie, R., O’Sullivan, J., Ashworth, J., Bhaskar, S., Williams, S., Biswas, S., Kehdi, E., Ramsden, S., Clayton-Smith, J., Black, G. and Lloyd, I. (2014) Personalized diagnosis and management of congenital cataract by next-generation sequencing. Ophthalmology, 121, 2124–2137. 56. Reis, L., Tyler, R. and Semina, E. (2014) Identification of a novel C-terminal extension mutation in EPHA2 in a familiy affected with congenital cataract. Mol. Vis., 20, 836–842. 5804 | Human Molecular Genetics, 2015, Vol. 24, No. 20 57. Abramoff, M., Magalhaes, P. and Ram, S. (2004) Image processing with ImageJ. Biol. Med. Phys. Biomed, 11, 36–42. 58. Larionov, A., Krause, A. and Miller, W. (2005) A standard curve based method for relative real time PCR data processing. BMC Bioinform., 6, 62. 59. Livak, K. and Schmittgen, T. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-(Delta Delta C(T)) method. Methods, 25, 402–408. 60. White, R., Sessa, A., Burke, C., Bowman, T., LeBlanc, J., Ceol, C., Bourque, C., Dovey, M., Goessling, W., Burns, C. and Zon, L. (2008) Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell, 2, 183– 189. 61. Kimmel, C., Ballard, W., Kimmel, S., Ullmann, B. and Schilling, T. (1995) Stages of embryonic development of the zebrafish. Dev. Dynam., 203, 253–310.