* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download macromolecules
Cell membrane wikipedia , lookup
Citric acid cycle wikipedia , lookup
Protein (nutrient) wikipedia , lookup
Endomembrane system wikipedia , lookup
Self-assembling peptide wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Bottromycin wikipedia , lookup
Peptide synthesis wikipedia , lookup
Protein adsorption wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Genetic code wikipedia , lookup
Cell-penetrating peptide wikipedia , lookup
Protein structure prediction wikipedia , lookup
Expanded genetic code wikipedia , lookup
List of types of proteins wikipedia , lookup
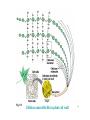
145 Zoo “Principles of Biology” Lectures ٢٠٠٩ Prepared by Dr. Nadia Al-Eisa Dr. Ola Alhabit Dr. Tahany Ayaad Dr. Promy Virk Dr. Intisar Alsuhaibani ١ Text Book Biology Seventh Edition by Campbell & Reece ٢ CHAPTER 5 THE STRUCTURE AND FUNCTION OF MACROMOLECULES Section A: Polymer principles 1. Most macromolecules are polymers • Cells join smaller organic molecules together to form larger molecules. • These larger molecules, macromolecules, may be composed of thousands of atoms. • The four major classes of macromolecules are: carbohydrates, lipids, proteins, and nucleic acids. ٣ 1. Most macromolecules are polymers • The macromolecules of carbohydrates, proteins, and nucleic acids form chainlike molecules called polymers. – Polymers consist of many similar or identical building blocks linked by covalent bonds. • The repeated units are small molecules called monomers. ٤ • The chemical mechanisms used to make and break polymers are similar for all classes of macromolecules. • Monomers are connected by covalent bonds via a dehydration reaction: – One monomer provides a hydroxyl group and the other provides a hydrogen atom to form water. – This process requires energy and is aided by enzymes. Fig. 5.2a ٥ • The covalent bonds connecting monomers in a polymer are disassembled by hydrolysis (hydration) reaction. – In hydrolysis as the covalent bond is broken a hydrogen atom and hydroxyl group from a split water molecule attaches where the covalent bond used to be. – Hydrolysis reactions dominate the digestive process, guided by specific enzymes. Fig. 5.2b ٦ (Carbohydrates, Lipids, Proteins and nucleic acids) Mono-mer Di-mer Poly-mer أﺣﺎدى ﺛﻨﺎﺋﻰ ﻋﺪﯾﺪ ٧ Carbohydrates - Fuel and Building Material • Carbohydrates include both sugars and polymers. • Monosaccharides or simple sugars are the simplest carbohydrates. • Disaccharides, double sugars, consist of two monosaccharides joined by a dehydration reaction. • Polysaccharides are polymers of many monosaccharides. ٨ 1. Sugars, the smallest carbohydrates serve as a source of fuel and carbon sources • Monosaccharides generally have molecular formulas that are some multiple of CH2O. – For example, glucose has the formula C6H12O6. – Most names for sugars end in -ose. • Monosaccharides have: 1. a carbonyl group (C=O) and 2. multiple hydroxyl groups (-OH). ٩ Monosaccharides are classified as following : A- Based on the location of C=O Aldoses: are the monosaccharides of carbonyle group (C=O) at the end of C chain (e.g. Glucose). Ketoses: are the monosaccharides of C=O carbonyle group within the C chain (e.g. Fructose). – Glucose (aldose) and fructose (a ketose), are structural isomers. B- Based on the number of C in the skeleton Triose (3 3C): e.g. Glyceraldehyde. Pentose (5 5C): e.g. Ribose. Hexose (6 6C): e.g. Glucose, Fructose and Galactose. Fig 5.3 ١٠ Fig 5.3 ١١ 2- Disaccharides Consisted of 2 monosaccharide molecules during a dehydration reaction ﺗﻔﺎﻋﻞ ﻧﺰع اﻟﻤﺎء. ﺗﻔﺎﻋﻞ. Sucrose (table sugar): sugar): consists of Glucose + Fructose. Fructose. ١٢ FIG. 5.5b 3. Polysaccharides, the polymers of sugars, have storage and structural roles • Polysaccharides are polymers of hundreds to thousands of monosaccharides joined by glycosidic linkages. • One function of polysaccharides is as an energy storage macromolecule that is hydrolyzed as needed. • Other polysaccharides serve as building materials for the cell or whole organism. • They are two types: ١٣ A. Storage Polysaccharides 1. Starch (in plants) is a storage polysaccharide consisted of thousands of glucose monomers. – – – Most monomers are joined by 1-4 linkages between the glucose molecules. Plants store starch within plastids, including chloroplasts. Animals, including human, have enzymes that can hydrolyze plant starch making glucose available for metabolism. ١٤ Fig. 5.7b Fig. 5.6 • There are two ring forms of glucose differ in whether the hydroxyl group attached to the number 1 carbon is fixed below (alpha glucose) or above (beta glucose) the ring plane. Fig. 5.7a ١٥ 2. Glycogen (in animals): • Animals store glucose in a polysaccharide called as glycogen in their liver and muscles. – Humans and other vertebrates store glycogen but only have about a one day supply. – Thus, it gives glucose when hydrolysed. Fig. 5.6b ١٦ B. Structural Polysaccharides • Structural polysaccharides form strong building materials. 1. Cellulose (in plants): • It is the building materials of the cell wall in plants. • It is a polymer of thousands of β glucose molecules. Fig. 5.7c ١٧ – Cellulose molecules are attached to each other by hydrogen bonds forming "Microfibrils" (fig. 5.8). – Human can not digest cellulose, as few organisms possess enzymes that can digest linkages of cellulose: Some bacteria and other microbes can digest cellulose (e.g. that live in the cow's stomach). ١٨ Fig. 5.8 Cellulose microfibrills in plant cell wall ١٩ 2. Chitin (in insects): • It is used in the exoskeletons of arthropods (including insects, spiders, and crustaceans). • Chitin is is consisted of thousands of glucose molecules with a Nitrogen atom in one end. • It is used to manufacture the surgical threads. Fig. 5.9 ٢٠ Carbohydrates No. of sugar molecules Monosaccharides Glucose & Fructose No. of C atoms Disaccharides Polysaccharides Maltose & Sucrose Location of C=O Triose (3C) Pentose (5 (5C) Hexose (6 (6C) Glyceraldehyde Ribose Glucose Aldose C=O on top Storage Starch (in plants) & Glycogen (in animals) Structural Cellulose (in plants) & Chitin (in insects) Ketose C=O in chain ٢١ CHAPTER 5 THE STRUCTURE AND FUNCTION OF MACROMOLECULES 5.3 Lipids are a diverse group of hydrophobic molecules ٢٢ Lipids • Lipids are macromolecules that they do not have polymers. • They all have little or no affinity for water. • Lipids are different in form and function. • They consist mostly of hydrocarbons smaller than polymer macromolecules. • The most important lipid families: fats, phospholipids and steroids. ٢٣ 1- Fats • Fats are not polymers. • They are large molecules assembled from smaller molecules by dehydration reactions. Structure of Fats • A fat molecule consists of 3 fatty acid molecules (16 – 18 C long) joind to glycerol by an ester linkage creating a triacylglycerol. Ester link Fig. 5.11a Dehydration Synthesis Fig. 5.11b ٢٤ • Fatty acids can be classified according to the location of double bonds: A. Saturated fatty acids: • • There are no C - C double bonds. Every C is linked with H (it is saturated with H). – Most animal saturated. fats are – Saturated fat are solid at room temperature. – A diet rich in saturated fats may cause cardiovascular disease (atherosclerosis). a. Saturated fats Fig. 5.12a ٢٥ B. Unsaturated fatty acids: – There are one or more C-C double bonds. – These double bonds are formed by the removal of H atoms from the carbon skeleton. • Plant and fish fats, known as oils, are liquid are room temperature. • Hydrogenated vegetable oils = +H Unsaturated fats saturated fats (to prevent lipid liquidity) Fig. 5.12b b. Unsaturated fats ٢٦ 2- Phospholipids Are major components of cell membranes • Phospholipids have 2 fatty acids attached to glycerol and a phosphate group at the third position. – The phosphate group carries a negative charge. – Additional smaller groups may be attached to the phosphate group. • phospholipids show ambivalent behavior toward water: – The fatty acid tails are hydrophobic, but the phosphate group and its attachments form a hydrophilic head. ٢٧ Fig. 5.13 • Phospholipids are major components of cell membranes: They are arranged in a bilayer (double layer) (Fig. 5.14). The hydrophilic heads of the molecules are on the outside of the bilayer, in contact with the aqueous solutions on both sides of the cell. The hydrophobic tails point toward the interior of the membrane, away from the water. The phospholipid bilayer forms a barrier between the cell and its external environment. water Hydrophilic head Hydrophobic tails Hydrophilic head water Fig. 5.14 ٢٨ Steroids • Steroids are lipids with a carbon skeleton consisting of 4 fused carbon rings. Different steroids are created by varying functional groups attached to the rings. Steroids include: Cholesterol, a component in animal cell membranes. sex hormones Fig. 5.15 ٢٩ Lipids Fats Phospholipids Bilayer of cell membrane Saturated Animal Fats Unsaturated Steroids Cholesterol & Sex Hormones Vegetable Fats Hydrogenation ٣٠ CHAPTER 5 THE STRUCTURE AND FUNCTION OF MACROMOLECULES 5.4 Proteins have many structures, resulting in a wide range of functions • Proteins account for more than 50% of the dry weight of most cells. • Humans have tens of thousands of different proteins, each with a specific structure and function. • The most important type of protein may be enzymes. • Each type of protein has a complex three-dimensional ٣١ shape or conformation. • protein is a polymer (polypeptide) constructed from 20 monomers (amino acids). • A polypeptide is a polymer of many amino acids linked by peptide bonds. • A protein consists of one or more polypeptides folded and coiled into a specific conformation. Fig. 5.19 ٣٢ Amino acids • Amino acids are organic molecules consisting of 4 components attached to a central carbon (a carbon): • These components include: • • • • a hydrogen atom a carboxyl group (–COOH) an amino group (–NH2) a variable R group (or side chain). Hydrogen atom Carboxyl group Amino group R Fig. The Structure of amino acid molecule Side chain ٣٣ • Differences in R groups produce the 20 different amino acids. • The amino acids are grouped according to the properties of their side chains as follows: 1. Nonpolar: the amino acids that have hydrophobic R groups. ٣٤ Fig. 5.17 2. Polar: the amino acids that have hydrophilic R groups. Fig. 5.17 3. Charged: the amino acids with functional groups that are charged (ionized) at cellular pH (7.2). – Fig. 5.17 Some R groups are bases, others are acids. ٣٥ Peptide bonds: • Amino acids are joined together when a dehydration reaction removes: a hydrogen a hydroxyl group (–OH) from the carboxyl (–COOH) + from the amino group (–NH2) of of one amino acid results the next amino acid a peptide bond (covalent bond) ٣٦ Fig. 5.18 • Repeating the dehydration process over and over creates a long polypeptide chain. – Each amino acid has two ends: – At one end there is a free amino group the (the N-terminus) – at the other there is a free carboxyl group the (the C-terminus). • The repeated sequence (N-C-C) is the polypeptide backbone. • Various R groups are attached to the backbone. • Polypeptides range in size from a few monomers to thousands. ٣٧ Amino acids Non-polar Polar Charged (Hydrophobic R group) (Hydrophilic R group) (Ionized functional group) Amino acids Peptide bond Polypeptide Conformation Protein Dehydration ٣٨