* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download 1 Chapter 5 Microbial Metabolism 2
Nicotinamide adenine dinucleotide wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Biosynthesis wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Phosphorylation wikipedia , lookup
Metalloprotein wikipedia , lookup
Metabolic network modelling wikipedia , lookup
Electron transport chain wikipedia , lookup
Photosynthesis wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Microbial metabolism wikipedia , lookup
Biochemistry wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Citric acid cycle wikipedia , lookup
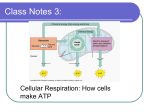
1 2 3 4 5 6 7 8 9 10 11 12 Chapter 5 Microbial Metabolism Catabolic and Anabolic Reactions 5-1 Define metabolism, and describe the fundamental differences between anabolism and catabolism. 5-2 Identify the role of ATP as an intermediate between catabolism and anabolism. Catabolic and Anabolic Reactions Metabolism: the sum of the chemical reactions in an organism Catabolic and Anabolic Reactions Catabolism: provides energy and building blocks for anabolism Anabolism: uses energy and building blocks to build large molecules Catabolic and Anabolic Reactions A metabolic pathway is a sequence of enzymatically catalyzed chemical reactions in a cell Metabolic pathways are determined by enzymes Enzymes are encoded by genes Check Your Understanding Distinguish catabolism from anabolism. 5-1 How is ATP an intermediate between catabolism and anabolism? 5-2 Enzyme 5-3 Identify the components of an enzyme. 5-4 Describe the mechanism of enzymatic action. 5-5 List the factors that influence enzymatic activity. 5-6 Distinguish competitive and noncompetitive inhibition. 5-7 Define ribozyme. Collision Theory The collision theory states that chemical reactions can occur when atoms, ions, and molecules collide Activation energy is needed to disrupt electronic configurations Reaction rate is the frequency of collisions with enough energy to bring about a reaction Reaction rate can be increased by enzymes or by increasing temperature or pressure Enzyme Components Biological catalysts Biological catalysts Specific for a chemical reaction; not used up in that reaction Apoenzyme: protein Cofactor: nonprotein component Coenzyme: organic cofactor Holoenzyme: apoenzyme plus cofactor 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 Important Coenzymes NAD+ NADP+ FAD Coenzyme A Enzyme Specificity and Efficiency The turnover number is generally 1 to 10,000 molecules per second Enzyme Classification Oxidoreductase: oxidation-reduction reactions Transferase: transfer functional groups Hydrolase: hydrolysis Lyase: removal of atoms without hydrolysis Isomerase: rearrangement of atoms Ligase: joining of molecules; uses ATP Factors Influencing Enzyme Activity Temperature pH Substrate concentration Inhibitors Factors Influencing Enzyme Activity Temperature and pH denature proteins Enzyme Inhibitors: Competitive Inhibition 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 Enzyme Inhibitors: Noncompetitive Inhibition Enzyme Inhibitors: Feedback Inhibition Ribozymes RNA that cuts and splices RNA Check Your Understanding What is a coenzyme? 5-3 Why is enzyme specificity important? 5-4 What happens to an enzyme below its optimal temperature? Above its optimal temperature? 5-5 Why is feedback inhibition noncompetitive inhibition? 5-6 What is a ribozyme? 5-7 Energy Production 5-8 Explain the term oxidation-reduction. 5-9 List and provide examples of three types of phosphorylation reactions that generate ATP. 5-10Explain the overall function of metabolic pathways. Oxidation-Reduction Reactions Oxidation: removal of electrons Reduction: gain of electrons Redox reaction: an oxidation reaction paired with a reduction reaction Oxidation-Reduction Reactions In biological systems, the electrons are often associated with hydrogen atoms Biological oxidations are often dehydrogenations The Generation of ATP ATP is generated by the phosphorylation of ADP Substrate-Level Phosphorylation Energy from the transfer of a high-energy PO4– to ADP generates ATP Oxidative Phosphorylation Energy released from transfer of electrons (oxidation) of one compound to 43 44 45 46 47 48 49 50 51 52 53 54 55 Oxidative Phosphorylation Energy released from transfer of electrons (oxidation) of one compound to another (reduction) is used to generate ATP in the electron transport chain Photophosphorylation Light causes chlorophyll to give up electrons Energy released from transfer of electrons (oxidation) of chlorophyll through a system of carrier molecules is used to generate ATP Check Your Understanding Why is glucose such an important molecule for organisms? 5-8 Outline the three ways that ATP is generated. 5-9 What is the purpose of metabolic pathways? 5-10 Carbohydrate Catabolism 5-11Describe the chemical reactions of glycolysis. 5-12Identify the functions of the pentose phosphate and EntnerDoudoroff pathways. 5-13Explain the products of the Krebs cycle. 5-14Describe the chemiosmotic model for ATP generation. 5-15Compare and contrast aerobic and anaerobic respiration. 5-16 Describe the chemical reactions of, and list some products of, fermentation. Carbohydrate Catabolism The breakdown of carbohydrates to release energy Glycolysis Krebs cycle Electron transport chain Glycolysis The oxidation of glucose to pyruvic acid produces ATP and NADH Preparatory Stage of Glycolysis 2 ATP are used Glucose is split to form 2 glucose-3-phosphate Energy-Conserving Stage of Glycolysis 2 glucose-3-phosphate are oxidized to 2 pyruvic acid 4 ATP are produced 56 57 58 59 60 61 62 63 64 65 66 67 68 69 2 glucose-3-phosphate are oxidized to 2 pyruvic acid 4 ATP are produced 2 NADH are produced Glycolysis Glucose + 2 ATP + 2 ADP + 2 PO4– + 2 NAD+ → 2 pyruvic acid + 4 ATP + 2 NADH + 2H+ Alternatives to Glycolysis Pentose phosphate pathway Uses pentoses and NADPH Operates with glycolysis Entner-Doudoroff pathway Produces NADPH and ATP Does not involve glycolysis Pseudomonas, Rhizobium, Agrobacterium Cellular Respiration Oxidation of molecules liberates electrons for an electron transport chain ATP is generated by oxidative phosphorylation Intermediate Step Pyruvic acid (from glycolysis) is oxidized and decarboxylated The Krebs Cycle Oxidation of acetyl CoA produces NADH and FADH2 The Electron Transport Chain A series of carrier molecules that are, in turn, oxidized and reduced as electrons are passed down the chain Energy released can be used to produce ATP by chemiosmosis A Summary of Respiration Aerobic respiration: the final electron acceptor in the electron transport chain is molecular oxygen (O2) Anaerobic respiration: the final electron acceptor in the electron transport chain is NOT O2 Yields less energy than aerobic respiration because only part of the Krebs cycle operates under anaerobic conditions Respiration Anaerobic Respiration 68 69 70 71 72 73 74 75 76 77 78 79 80 81 82 Respiration Anaerobic Respiration Carbohydrate Catabolism Carbohydrate Catabolism Energy produced from complete oxidation of one glucose using aerobic respiration Carbohydrate Catabolism ATP produced from complete oxidation of one glucose using aerobic respiration Carbohydrate Catabolism 36 ATPs are produced in eukaryotes Fermentation Any spoilage of food by microorganisms (general use) Any process that produces alcoholic beverages or acidic dairy products (general use) Any large-scale microbial process occurring with or without air (common definition used in industry) Fermentation Scientific definition: Releases energy from oxidation of organic molecules Does not require oxygen Does not use the Krebs cycle or ETC Uses an organic molecule as the final electron acceptor Fermentation Alcohol fermentation: produces ethanol + CO2 Lactic acid fermentation: produces lactic acid Homolactic fermentation: produces lactic acid only Heterolactic fermentation: produces lactic acid and other compounds Check Your Understanding What happens during the preparatory and energy-conserving stages of glycolysis? 5-11 What is the value of the pentose phosphate and Entner-Doudoroff pathways if they produce only one ATP molecule? 5-12 What are the principal products of the Krebs cycle? 5-13 83 84 85 86 87 88 89 90 91 92 93 94 95 96 97 What is the value of the pentose phosphate and Entner-Doudoroff pathways if they produce only one ATP molecule? 5-12 What are the principal products of the Krebs cycle? 5-13 How do carrier molecules function in the electron transport chain? 5-14 Compare the energy yield (ATP) of aerobic and anaerobic respiration. 5-15 List four compounds that can be made from pyruvic acid by an organism that uses fermentation. 5-16 Lipid and Protein Catabolism 5-17Describe how lipids and proteins undergo catabolism. 5-18Provide two examples of the use of biochemical tests to identify bacteria in the laboratory. Protein Catabolism Protein Catabolism Biochemical Tests Used to identify bacteria Check Your Understanding What are the end-products of lipid and protein catabolism? 5-17 On what biochemical basis are Pseudomonas and Escherichia differentiated? 5-18 Photosynthesis 5-19Compare and contrast cyclic and noncyclic photophosphorylation. 5-20Compare and contrast the light-dependent and light-independent reactions of photosynthesis. 5-21Compare and contrast oxidative phosphorylation and photophosphorylation. Photosynthesis Photo: conversion of light energy into chemical energy (ATP) Light-dependent (light) reactions Synthesis: Carbon fixation: fixing carbon into organic molecules Light-independent (dark) reaction: Calvin-Benson cycle Photosynthesis Light-independent (dark) reaction: Calvin-Benson cycle 96 97 98 99 100 101 102 103 104 105 106 107 108 109 Photosynthesis Oxygenic: Anoxygenic: Cyclic Photophosphorylation Noncyclic Photophosphorylation Calvin-Benson Cycle Check Your Understanding How is photosynthesis important to catabolism? 5-19 What is made during the light-dependent reactions? 5-20 How are oxidative phosphorylation and photophosphorylation similar? 5-21 A Summary of Energy Production 5-22Write a sentence to summarize energy production in cells. Check Your Understanding Summarize how oxidation enables organisms to get energy from glucose, sulfur, or sunlight. 5-22 Metabolic Diversity among Organisms 5-23Categorize the various nutritional patterns among organisms according to carbon source and mechanisms of carbohydrate catabolism and ATP generation. Chemotrophs Use energy from chemicals Chemoheterotroph 110 111 Energy is used in anabolism Chemotrophs Use energy from chemicals Chemoautotroph, Thiobacillus ferrooxidans Energy is used in the Calvin-Benson cycle to fix CO2 Phototrophs Use light energy Photoautotrophs use energy in the Calvin-Benson cycle to fix CO2 Photoheterotrophs use energy 112 113 114 115 116 117 118 119 120 121 122 Metabolic Diversity among Organisms Check Your Understanding Almost all medically important microbes belong to which of the four aforementioned groups? 5-23 Metabolic Pathways of Energy Use 5-24Describe the major types of anabolism and their relationship to catabolism. Check Your Understanding Where do amino acids required for protein synthesis come from? 5-24 122 123 124 125 126 Check Your Understanding Where do amino acids required for protein synthesis come from? 5-24 The Integration of Metabolism 5-25Define amphibolic pathways. The Integration of Metabolism Amphibolic pathways: metabolic pathways that have both catabolic and anabolic functions Check Your Understanding Summarize the integration of metabolic pathways using peptidoglycan synthesis as an example. 5-25