* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Energy Systems
Metalloprotein wikipedia , lookup
Electron transport chain wikipedia , lookup
Photosynthesis wikipedia , lookup
Lactate dehydrogenase wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Microbial metabolism wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Citric acid cycle wikipedia , lookup
Biochemistry wikipedia , lookup
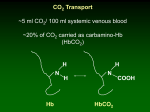
Objectives • Bioenergetics ~ energy transfer a) Within the body b) During exercise Energy Systems • ATP regeneration • Sources of energy during exercise: a) Anaerobic b) Aerobic Energy Transfer • Direct transfer of chemical energy required for all forms of biological work a) Energy = the capacity for work Bioenergetics 9 Dynamic state related to change 9 As work load increases, energy transfer increases • Bioenergetics & Thermodynamics a) Process of converting food stuffs into “harnessed” energy 1st Law of Thermodynamics • Energy is neither created nor destroyed, but is transformed from one form to another a) As the body undergoes transformations, energy is changed from one form to another 9 Conservation of energy Energy in food Heat ATP Potential & Kinetic Energy Total (heat) energy of a system includes both: 1. Potential energy • Bound in a specific form 2. Kinetic energy Mechanical Chemical • Harnessing of potential energy (energy of motion) • Biosynthesis results from harnessing energy 9 Individual atoms are joined to synthesize biologic compounds 1 Energy Release & Conservation • Exergonic reactions (-∆G) a) Physical or chemical release of energy Energy Release & Conservation • Endergonic reactions a) Chemical processes that store or absorb energy 9 ‘Gibbs free energy’ b) Measurement of ‘free energy’ G = free energy G = H - TS • Processes may be coupled a) Exergonic + Endergonic ∆G = ∆ H - T ∆ S H = Enthalpy (potential energy) T = Absolute temperature (ºC + 273) S = Unavailable energy due to randomness ex: H2 + O H2O ~ -∆G 68kcal/mol H2 + O H2O ~ +∆G 68kcal/mol Examples of ∆G for Important Molecules 2nd Law of Thermodynamics • All reactions proceed in the direction of: a) Increased entropy b) The release of free energy • The more –∆G, the greater the release of free energy • Direction & amount of free energy may be modified by altering a) Substrate concentration b) Product concentration Glucose-6-phosphate -3.3 ∆G Glucose-1-phosphate -5.0 ∆G ATP -7.3 ∆G Creatine Phosphate -10.3 ∆G Rate of Bioenergetics • Enzymes function as: a) Biological Catalysts – speed up chemical reactions without being involved in the reaction or altering the free energy release b) Couplers – provide means to couple reactions c) Regulators of metabolism • Factors influencing enzyme function: a) pH b) Temperature c) Availability of substrate and enzyme 2 Major affects on enzyme activity Figure 5.7 Enzymes (cont.) • Mode of action a) Lock and key mechanism (active site) b) Enzyme-substrate complex 9 After entering active site catalyzes reaction & end product produced • Enzymes increase or decrease the likelihood of a reaction occurring a) Allosteric modification – can be activated or inhibited 9 PFK (glycolysis) & Phosphorylase (glycogenolysis) Figure 5.8 Alterations in enzyme concentration Coenzymes • Complex, non-protein organic substance a) Iron b) Zinc c) B vitamins • Require less specificity than enzymes a) Affects various numbers of reactions b) May serve as a temporary carrier 9 Nicotinamide adenine dinucleotide (NAD+) 9 Flavin adenine dinucleotide (FAD) 3 Hydrolysis & Condensation Rx 1. Hydrolysis reactions • Catabolizes complex organic molecules (CHO, lipids, proteins) 9 Specific enzymes for each molecule • Splits chemical bonds by adding H+ and OH9 Digestive enzymes 2. Condensation reactions • Synthesis of molecules or anabolic process • Reverse of hydrolysis 9 Peptide bonds Figure 5.9 Electrons, protons & oxidation-reduction reactions • Electrons a) Negatively charged subatomic particles circulating around the atom nucleus b) Essential for atoms to form covalent (sharing) bonds c) During many chemical reactions 9 Electrons are either removed or added to molecules • Molecules that lose 1 or more electrons are oxidized, whereas molecules that gain electrons are reduced Figure 5.9 • Oxidation involves the loss of electrons, & reduction involves the gaining of electrons • Reactions occur together and often termed oxidation-reduction or redox reactions A:e + B A + B:e example: Pyruvate + NADH + H+ lactate + NAD+ (Reduced) (Oxidized) Q – which molecule was reduced & which oxidized in the direction of lactate production? Figure 5.11 4 Bioenergetics Review • Law’s of Thermodynamics • Exergonic & endergonic rx Energy Transfer a) Potential & kinetic energy • Enzymes & coenzymes • Hydrolysis, condensation & redox reactions Adenosine Triphosphate (ATP) ATP + H2O ATPase ADP + Pi ∆G = -7.3kcal/mol Food Energy Figure 6.2 ATP – Energy Currency of the Cell ATP Regeneration • Design & function of skeletal muscle metabolism is to meet rapidly meet the ATP demand • Skeletal muscle can produce the necessary ATP for muscle contraction from 1 or a combination of three metabolic reactions/pathways: ex: Muscle contraction can ↑ cellular ATP demand by 100 fold 9 Could deplete resting [ATP] in as little as 2 – 3 seconds of intense exercise • Skeletal muscle has sensitive biochemical controls of metabolic pathways involving the sudden activation and inhibition of specific enzymes 1. Phosphagen System 2. Glycolysis 3. Mitochondrial Respiration 5 Anaerobic vs. Aerobic Immediate Sources of ATP (Anaerobic) Energy Reservoirs Energy Reservoirs (cont.) 1. Phosphocreatine 2. Andenylate Kinase • Most immediate means for ATP regeneration • CrP + ADP + H+ ATP + Cr • Actually 2 “coupled” reactions CrP Cr + Pi ADP + H+ + Pi exergonic ADP + ADP ATP endergonic • Responsive to immediate changes in ADP & ATP concentrations Important By-products of the Phosphogen Systems • PCr hydrolysis and adenylate kinase reaction generate: a) Pi b) AMP c) ADP Reforms ATP using two ADP molecules 9 Results in ATP and AMP 9 Adenylate kinase drives this reaction 9 Requires only 1 enzyme, creatine kinase (CK) CK • AK ATP + AMP AMP acts as an important regulator 9 Activator of the allosteric enzymes phosphorylase (glycogenolysis) and phosphofructokinase (PFK) Exercise Specifics • ATP – PCr system a) Provides ATP for short-term, high-intensity movements & is rapidly depleted By-products • By-products stimulate: a) Glycogenolysis b) Glycolysis c) Respiratory pathways of mitochondria What type of exercises include these systems? 6 Glycogen & Glycogen Stores 1. Liver Glycogenolysis & Glycolysis • Reservoir for blood glucose & brain 2. Muscle • Does not release to blood (only for muscle metabolism) Biochemically efficient – no net ATP cost Glucose requires one ‘extra’ ATP Glycogenolysis • Catabolism of glycogen requires a) Removal of glucose units from glycogen b) Addition of Pi 9 End product – glucose-6-phosphate • Pi & Ca2+ major regulators of glycogenolysis a) Epinephrine also ↑ • Major enzyme required is phosphorylase Glycolysis • Glucose (glucose-6-phosphate) → pyruvate • Rapid rate of net 2 ATP • Produces 2 NADH + 2 H+ • Provides substrate for other pathways a) Pyruvate b) Lactate • Transported into the cell by GLUT proteins 7 • Pyruvate reduction to lactate: a) Lactate dehydrogenase (LDH) Pyruvate + NADH + H+ LDH lactate + NAD+ b) Depends on availability of NADH 9 NADH/NAD+ (redox potential) Via glycerolphosphate shuttle & malateaspartate shuttle Via glycerolphosphate shuttle & malateaspartate shuttle c) Reduction of pyruvate to lactate helps to buffer the solution 9 ‘absorbing’ excess protons (H+) Sport Application • Glycolysis resulting in lactate formation (lactic lactic acid system) system • Lactate accumulation a) Blood Lactate Threshold (LT) 9 Lactate production exceeds clearance 9 Average for untrained = ~ 55% max aerobic capacity (Davis JA et al., JAP 1979) 9 Training increases lactate threshold Figure 6.12 Sport Application (cont.) • Lactate-producing capacity increases with anaerobic training a) Increased intramuscular glycogen stores 9 Allows for increased glycolysis b) Increased glycolytic enzymes 9 Training increases ~ 20% c) Increased ability to recruit Type IIb fibers Figure 7.2 8 Lactate Is Not a Waste Product! 1. Lactate shuttle • Converted to pyruvate and oxidized as an energy source in another cell 2. Gluconeogenesis • Converted back to glucose in the liver in Cori Cycle Figure 6.13 Sources of ATP (Aerobic & Steady-State) Pyruvate Dehydrogenase Complex acetyl CoA → TCA cycle • Pyruvate entry into mitochondria is converted to acetyl CoA by a series of linked enzymes known collectively as pyruvate dehydrogenase • Combined products of the TCA cycle from acetyl CoA are: Pyruvate dehydrogenase complex Pyruvate + NAD+ + CoA Acetyl CoA + NADH + H+ + CO2 • Glycolysis to TCA cycle • Irreversible step a) 2 CO2 b) 1 ATP c) 3 NADH + H+ d) 1 FADH2 Support ATP regeneration during oxidative phosphorylation • All CO2 produced in energy metabolism are accounted for from pyruvate dehydrogenase reaction and 2 reactions of the TCA cycle 9 TCA key points • TCA cycle is ‘ultimate furnace’ a) Amino acids b) Glucose c) Fatty acids d) Ketones acetyl CoA • Main electron transferring pathway (redox reactions – energy conserved in NADH & FADH) Figure 6.15 TCA key points (cont.) Electron Transport Chain (ETC) • Each ‘turn of cycle’: one acetyl-CoA enters and 2 CO2 leave • Involves the biochemical use of O2 to regenerate ATP in mitochondria • Synthesis of citrate which is an inhibitor for glycolysis at PFK a) Mainly during rest and post exercise recovery • Oxaloacetate is regenerated with each turn • ETC a) Series of electron receivers located along the inner mitochondrial membrane that sequentially receive and transfer electrons to the final electron receiver – oxygen • Consumption of oxygen, and formation of water and ATP during the ETC is termed oxidative phosphorylation 10 Summary Summary (cont.) – Regulation of Energy Metabolism • Overall energy state dictates the direction of metabolic pathways • Rate-limiting modulators: 1. 2. 3. 4. 5. 6. ATP ADP cAMP NAD Calcium pH • It is the relative concentrations that are important: NADH/NAD+ & ATP/ADP Sport Application & Concepts Oxygen Consumption During Exercise: Application & Measurement of Energy Metabolism • Oxygen consumption (VO2) a) Pulmonary oxygen uptake b) Oxygen is measured at lung, not tissues (debatable) c) Steady-state 9 When oxygen demand is met by oxygen delivery 9 Blood lactate doesn’t accumulate 9 Exercise may continue at this rate until limitations other than oxygen alter performance Sport Application & Concepts (cont.) Oxygen Deficit: • As exercise begins: a) Oxygen demand increases immediately b) Oxygen consumption lags behind • Oxygen deficit a) Quantitative expression of difference between oxygen consumed and the amount that would have been consumed had steady state been reached right from the start. Figure 7.3 11 Sport Application & Concepts (cont.) Sport Application & Concepts (cont.) Oxygen Deficit (cont.): Maximal Oxygen Consumption (VO2max): • Trained Individuals • Maximal volume of oxygen one can consume a)Reach steady-state more rapidly b)Have a smaller oxygen deficit 9 More rapid increase in cardiac output 9 Larger percentage of blood directed to active muscle 9 Training induced cellular adaptations 1. Increased capillary density 2. Increased number mitochondria 3. Increased oxidative enzymes 9 Maximal oxygen uptake 9 Maximal aerobic power 9 Aerobic capacity • Provides a quantitative measure of capacity for aerobic ATP resynthesis Muscle Fiber Types • Fast- and slow-twitch muscle fibers a) Slow-twitch = Type I 9 Highest aerobic capacity 9 Lowest glycolytic capabilities b) Fast-twitch = Type II 9 Type IIa: Medium glycolytic and aerobic capabilities 9 Type IIb: Highest glycolytic capacity & lowest aerobic capacity Figure 7.5 O2 Consumption During Recovery • Metabolic dynamics of recovery oxygen consumption • Excess postexercise oxygen consumption (EPOC): Type II Type I a) Oxygen cost of adjustments in: 9 9 9 9 9 Ventilation Circulating hormones Blood circulation Temperature O2 reloading with muscle Speed of recovery depends on recovery mode 12 Energy from Fat Energy Release From Fat • Adipocytes a) Site of fat storage and mobilization b) Fat is stored primarily as triglycerides • Mobilization a) First step in utilizing fatty acids is Lipolysis b) Triglycerides are split into: 9 Fatty acids 9 Glycerol c) Hormone Sensitive Lipase (HSL) drives lipolysis Transport and Uptake of Fatty Acids • Fatty acids from Lipolysis are FFA a) Bound to Albumin for transport in plasma • FFA are taken up by muscle cells a) FFA are activated to fatty Acyl CoA b) Acyl CoA binds to Carnitine for transport into mitochondria c) Carnitine Acyltransferase drives this reaction 13 Fatty Acids From Lipoproteins • Lipoproteins also transport triglycerides Circulating triglycerides • Lipoprotein Lipase (LPL) catalyzes hydrolysis of these triglycerides • LPL is located on surface of surrounding capillaries Circulating triglycerides Oxidation of fat w/in muscle • Beta Oxidation a) Cleaves two-carbon compounds from fatty Acyl CoA molecule b) Two-carbon acetyl groups enter Citric Acid Cycle c) Oxidation produces NADH • Fate of Glycerol a) Conversion to Pyruvate via glycolytic action b) Gluconeogenesis 9 Converted to Glucose in Liver Hormonal Effects • Lipolysis is stimulated by: a) Epinephrine b) Norepinephrine c) Glucagon d) Growth Hormone Energy from Protein • Intracellular mediator a) cAMP activates hormone sensitive lipase 14 Branched-chain Amino Acids • AA play contributory role as energy substrates during endurance and heavy resistance exercises • Requires removal of nitrogen from AA a) Liver – deamination b) Muscle – transamination • Depend on enzyme concentration (training) Figure 1.25 15