* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Test Information Sheet
Genetic engineering wikipedia , lookup
Dominance (genetics) wikipedia , lookup
Tay–Sachs disease wikipedia , lookup
Genetic testing wikipedia , lookup
Fetal origins hypothesis wikipedia , lookup
Nutriepigenomics wikipedia , lookup
Epigenetics of neurodegenerative diseases wikipedia , lookup
Gene expression programming wikipedia , lookup
Pharmacogenomics wikipedia , lookup
No-SCAR (Scarless Cas9 Assisted Recombineering) Genome Editing wikipedia , lookup
Genome evolution wikipedia , lookup
Gene therapy of the human retina wikipedia , lookup
Gene therapy wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Genome (book) wikipedia , lookup
BRCA mutation wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Cell-free fetal DNA wikipedia , lookup
Designer baby wikipedia , lookup
Population genetics wikipedia , lookup
Koinophilia wikipedia , lookup
Neuronal ceroid lipofuscinosis wikipedia , lookup
Oncogenomics wikipedia , lookup
Saethre–Chotzen syndrome wikipedia , lookup
Microevolution wikipedia , lookup
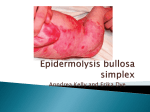
GeneDx 207 Perry Parkway Gaithersburg, MD 20877 Phone: 301-519-2100 Fax: 301-519-2892 E-mail: [email protected] www.genedx.com Test Information Sheet COL7A1 Gene Analysis in Dystrophic Epidermolysis Bullosa (DEB) Mendelian Inheritance in Man Numbers: Dystrophic Epidermolysis Bullosa (120120), Hallopeau Siemens form of DEB (226600), Pasini form of DEB (131750), pretibial form of DEB (131850), pruriginosa form (604129), epidermolysis bullosa with congenital absence of skin and deformity of nails (132000), transient bullous dermolysis of the newborn (131705) Clinical features: In this clinical type of EB, blistering usually begins in the neonatal period and may continue throughout life or may be transient (transient bullous dermolysis of the newborn). Blisters may be generalized and include oral and esophageal lesions in the severest form (Hallopeau-Siemens) or may be localized to the elbows and knees, and/or hands and feet in the milder forms. In addition, dystrophic nails are also often present. Dystrophic EB is not usually lethal but in the severest cases infants may succumb to infection or other complications. The lifetime risk of squamous cell carcinoma in patients with the Hallopeau-Siemens form is over 90%. In affected individuals the tissue separation (blister) occurs below the lamina densa. Anchoring fibrils may be reduced or absent. Collagen VII staining may be reduced or absent in the more severe forms or may appear relatively normal in the milder forms. Inheritance pattern: Dystrophic EB is due to mutations in only the COL7A1 gene, although there is significant variability in the severity of the phenotype in different individuals. DEB may have either an autosomal recessive or autosomal dominant inheritance pattern, depending upon the mutation and its location. The recurrence risk for couples with a child affected with the recessive form is 25%. In the dominant forms recurrence risk is 50% to offspring of an affected parent, although rarely unaffected carrier individuals have been observed (Pfendner personal communication). The recurrence risk to unaffected extended family members is low in the absence of consanguinity. Many de novo dominant mutations have been reported and the determination of recurrence risk is dependent upon identification of the mutation(s) and inheritance pattern. Risk of recurrence in subsequent pregnancies after a child is born with a de novo dominant mutation is 2-5%. Reasons for referral: 1. Confirmation of a clinical diagnosis 2. Genetic counseling and recurrence risk calculation 3. Prenatal diagnosis in families with an affected child and defined mutation Test method: Sequencing of the COL7A1 genes is offered. Using genomic DNA obtained from the submitted biological material, bi-directional sequence of the coding region and splice junctions of the COL7A1 gene (118 coding exons) is analyzed where over 90% of mutations have been identified in DEB patients with clinically and histologically confirmed disease. Mutation found in the first person of a family to be tested is confirmed by repeat analysis using sequencing, restriction fragment analysis, or another appropriate method. Sequencing of specific exons may be recommended for specific ethnic groups or phenotypes, especially in confirmed or suspected dominant dystrophic EB, after consultation with the EB specialist at GeneDx ([email protected], 610,-574-3479). Deletion of specific exons has been reported rarely. Testing for deletions is available after full COL7A1 gene screening at GeneDx, where one or both mutations are not detected and biopsy confirming the diagnosis of DEB has been performed. Information Sheet on Dystrophic Epidermolysis Bullosa Page 1 of 2 GeneDx Revision Date: 05/2013 Test sensitivity: Sequencing of the COL7A1 gene is expected to identify mutations in greater than 90% of patients with clinical and histologic features of DEB. The underlying genetic cause of DEB in the remaining cases may be due to mutations in the promoter, deep into introns, or large deletions not identifiable by our methods. A skin biopsy studied with appropriate collagen VII antibodies and/or electron microscopy to confirm the diagnosis of DEB is strongly recommended prior to pursuing genetic analysis. Mutation spectrum: All types of mutations have been reported in the COL7A1 gene and result in reduced or absent collagen VII protein. Generally, the severest forms of the disease are the result of nonsense mutations or out of frame insertions or deletions on both alleles while milder forms may be due to splicing mutations or missense mutations on one or both alleles. However, numerous exceptions have been reported. Specimen Requirements and Shipping/Handling: Blood: A single tube with 1-5 mL whole blood in EDTA. Ship overnight at ambient temperature, using a cool pack in hot weather. Specimens may be refrigerated for 7 days prior to shipping. Buccal Brushes: As an alternative to blood, use a GeneDx buccal kit (others not accepted). Submit by mail. Buccal brushes are not accepted on children under 6 months of age. Prenatal Diagnosis: For prenatal testing for a known mutation in the COL7A1 gene, please refer to the specimen requirements table on our website at: http://www.genedx.com/test-catalog/prenatal/. Ship specimen overnight at ambient temperature, using a cool pack in hot weather. Required Forms: Sample Submission (Requisition) Form – complete all pages Payment Options Form or Institutional Billing Instructions For test codes, prices, CPT codes, and turn-around-times, please refer to the “Dystrophic Epidermolysis Bullosa” page on our website: www.genedx.com References: 1. Jarvikallio A et al., 1997 Hum Mutat 10:338. 2. Rouan F, et al., 1998 J Invest Dermatol 111:1210. 3. Fine J-D et al., 2000 J Am Acad Dermatol 42:1051-106 4. Anton-Lamprecht & Gedde-Dahl, 2002. Epidermolysis bullosa. In: Rimoin DL, Connor JM, Pyeritz RE, Korf BR, eds. Principles and Practice of Medical Genetics. 4th ed. London: Churchill-Livingston 2002, 3810-3897. 5. Varki et al., 2007 J Med Genet 44: 181-192. Information Sheet on Dystrophic Epidermolysis Bullosa Page 2 of 2 GeneDx Revision Date: 05/2013