* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download LETTERS Transcription and Translation are
Survey
Document related concepts
Transcript
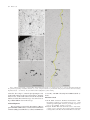
LETTERS Transcription and Translation are Coupled in Archaea Sarah L. French,* Thomas J. Santangelo, Ann L. Beyer,* and John N. Reeve *Department of Microbiology, University of Virginia Health System; Department of Microbiology, The Ohio State University Polysomes have been visualized by electron microscopy attached directly to dispersed strands of genomic DNA extruded from lysed cells of the hyperthermophilic archaeon Thermococcus kodakaraensis. These complexes are consistent with transcription and translation being coupled in this Archaeon, with translation of transcripts being initiated before the transcript is complete. The presence or absence of a nuclear membrane has historically been used as a taxonomic feature to divide all life into 2 groups, eukaryotes or prokaryotes, respectively. However, there is now very substantial molecular support for 3 primary phylogenetic domains (Woese 2000), and the classical definition of a prokaryote applies to both Bacteria and Archaea. Given that Bacteria and Archaea do not constitute one coherent phylogenetic lineage, that the definition based on the absence of a feature is scientifically invalid, and that prokaryotic is often inaccurately used as meaning bacterial, Pace (2006) recommended that the term prokaryote no longer be used. In response, Martin and Koonin (2006) pointed out that the absence of a nuclear membrane does provide the opportunity for ribosomes to bind to a transcript and so initiate translation before the transcript is complete, and that this could be a positive feature of being a prokaryote. Coupling of transcription and translation has been documented and studied extensively in Bacteria, where it provides the molecular basis for regulation of gene expression by attenuation (Grundy and Henkin 2006). The close association of translating ribosomes with the advancing bacterial RNA polymerase can affect the structure of the growing transcript and so has the potential to influence intrinsic transcription termination (Pan and Sosnik 2006) and can also prevent loading of the transcription termination factor rho (q). In Bacteria, when translation by pursuing ribosomes is halted or significantly delayed, continued transcription is usually limited resulting in the polarity observed in expression of promoter-distal genes in multigene operons (Yanofsky 2003). It seems reasonable to predict that Archaea will also have exploited the regulatory opportunities offered by coupling transcription and translation, but there is no published experimental support for this prediction. Archaeal genome sequences and northern blot results do argue convincingly that many archaeal genes are cotranscribed as members of multigene operons, but now with over 30 archaeal genomes sequenced only one putative attenuator has been identified, and this seems likely to be regulated by a riboswitch rather than by a translating ribosome (Rodionov et al 2003). Furthermore, archaeal genomes do not encode detectable homologues of q nor have functionally equivalent termination factors been identified, and polarity has not been demonKey words: Archaea, prokaryote, coupled transcription, polysome, electron microscopy. E-mail: [email protected]. Mol. Biol. Evol. 24(4):893–895. 2007 doi:10.1093/molbev/msm007 Advance Access publication January 20, 2007 Ó The Author 2007. Published by Oxford University Press on behalf of the Society for Molecular Biology and Evolution. All rights reserved. For permissions, please e-mail: [email protected] strated in Archaea. Furthermore, although archaeal RNA polymerases do exhibit intrinsic termination, they terminate in response to DNA template sequences without an apparent requirement for nascent transcript folding (Santangelo and Reeve 2006). Given the importance of coupled transcription and translation in Bacteria, and the surprising lack of evidence for such coupling in Archaea, we repeated the classic chromatin spreading experiment of Miller et al. (1970) using archaeal cells of the marine hyperthermophile Thermococcus kodakaraensis (Atomi et al. 2004). As shown in figure 1a, T. kodakaraensis cells are quasi-spherical and multiflagellate, and they lyse spontaneously when resuspended in water. Electron microscopic examination of chromatin extruded from such cells clearly revealed the presence of polysomes containing up to 20 adjacent ribosomes attached directly to dispersed strands of the archaeal genomic DNA (figs. 1b–d). The polysome patterns were consistent with the sequential direct binding of ribosomes to nascent mRNAs. Polysomes were only rarely seen separated from DNA. Given these observations, translation of mRNAs is apparently initiated in T. kodakarensis before the transcript is complete, and it seems likely that this is also the case in most if not all Archaea. In this regard, it is noteworthy that many but not all archaeal genes are preceded by sequences consistent with ribosome-binding sites, but that some archaeal transcripts have no leader sequence (Torarinsson et al. 2005). When and how ribosomes attach to these transcripts is clearly a puzzle, and experiments are also now needed to determine if the presence of a translating ribosome has any influence on transcription termination by the advancing archaeal RNA polymerase. Given the results obtained, prokaryotes could be defined as species in which translation of transcripts can be initiated before the transcript is complete (Martin and Koonin 2006). Although this does define prokaryotes positively at an organizational grade, it provides no clear support for any particular phylogenetic relationship of prokaryotes to eukaryotes. Methods Aliquots (1 ml) were removed from cultures of T. kodakaraensis strain KW128 growing exponentially in rich media (MA-YT-S°) at 82 °C (Sato et al 2005). The time between sampling and cell lysis was minimized by rapid sedimentation (microfuge centrifugation for 7 s) and immediate resuspension in water or in 0.025% Triton X100 (pH 9). Samples of the resuspended cells were centrifuged onto carbon-coated 300-mesh copper electron microscopy grids, 894 French et al. FIG. 1.—Electron micrographs of genomic DNA extruded from lysed Thermococcus kodakaraensis cells. (a) A lysed cell showing flagella and the spontaneous spreading of extruded chromatin. (b, d, and c) Dispersed strands of genomic DNA with polysomes attached shown at increasing magnifications (bar 5 0.2 microns). In (d), the DNA strand is highlighted by yellow shading. stained for 30 s using 1% ethanolic phosphotungstic acid, washed with ethanol, stained for 1 min with 1% ethanolic uranyl acetate, washed with ethanol, and air dried (Osheim and Beyer 1989). Electron microscopy was carried out using a JEOL 100CX electron microscope. Acknowledgments This research was supported by Department of Energy grant DE-FG02-87ER13731 (to J.N.R.), National Institutes of Health (NIH) grants GM53185 (to J.N.R.) and GM63952 (to A.L.B.), and NIH fellowship 1F32-GM073336-01 (to T.J.S.). Literature Cited Atomi H, Fukui T, Kanai T, Morikawa M, Imanaka T. 2004. Description of Thermococcus kodakaraensis sp. nov., a well studied hyperthermophilic archaeon previously reported as Pyrococcus sp. KOD1. Archaea. 1:263–267. Grundy FJ, Henkin TM. 2006. From ribosome to riboswitch: control of gene expression in bacteria by RNA structural rearrangements. Crit Rev Biochem Mol Biol. 41:329–338. Transcription and Translation Coupled in Archaea 895 Martin W, Koonin EV. 2006. A positive definition of prokaryotes. Nature. 442:868. Miller OL Jr, Hamkalo BA, Thomas CA Jr. 1970. Visualization of bacterial genes in action. Science. 169:392–395. Osheim YN, Beyer AL. 1989. Electron microscopy of ribonucleoprotein complexes on nascent RNA using the Miller chromatin spreading method. Methods Enzymol. 180:481–509. Pace NR. 2006 Time for a change. Nature. 441:289. Pan T, Sosnick T. 2006. RNA folding during transcription. Annu Rev Biophys Biomol Struct. 35:161–175. Rodionov DA, Vitreschak AG, Mironov AA, Gelfand MS. 2003. Regulation of lysine biosynthesis and transport genes in bacteria: yet another RNA riboswitch? Nucleic Acids Res. 31:6748–6757. Santangelo TJ, Reeve JN. 2006. Archaeal RNA polymerase is sensitive to intrinsic termination directed by transcribed and remote sequences. J Mol Biol. 355:196–210. Sato T, Fukui T, Atomi H, Imanaka T. 2005. Improved and versatile transformation system allowing multiple genetic manipulations of the hyperthermophilic archaeon Thermococcus kodakaraensis. Appl Environ Microbiol. 71:3889–3899. Torarinsson E, Klenk H-P, Garrett RA. 2005. Divergent transcriptional and translational signals in Archaea. Environ Microbiol. 7:47–54. Woese CR. 2000. Interpreting the universal phylogenetic tree. Proc Natl Acad Sci USA. 97:8392–8396. Yanofsky C. 2003. Using studies on tryptophan metabolism to answer basic biological questions. J Biol Chem. 278:10859–10878. William Martin, Associate Editor Accepted January 3, 2007