* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download electron transport chain
Metalloprotein wikipedia , lookup
Photosynthesis wikipedia , lookup
Phosphorylation wikipedia , lookup
Mitochondrion wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Biochemistry wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Microbial metabolism wikipedia , lookup
Citric acid cycle wikipedia , lookup
Light-dependent reactions wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
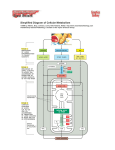
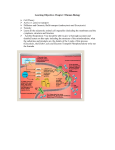
Electron transport chain Cellular respiration is a series of reactions that: -are oxidations – loss of electrons -are also dehydrogenations lost electrons are accompanied by hydrogen what is actually lost is a hydrogen atom (1 electron, 1 proton). • A hydrogen atom is equivalent to a hydrogen ion plus an electron H = H+ + e-electrons carry energy from one molecule to another. -electrons are shuttled through electron carriers to a final electron acceptor aerobic respiration: final electron receptor is oxygen (O2) NAD+ is an electron carrier. -NAD accepts 2 electrons + 1 proton to become NADH. -the reaction is reversible The goal of respiration is to produce ATP. - energy is released from oxidation reaction in the form of electrons - electrons are shuttled by electron carriers (e.g. NAD+) to an electron transport chain - electron energy is converted to ATP at the electron transport chain Oxidation of Glucose Cells are able to make ATP via: 1. substrate-level phosphorylation – transferring a phosphate directly from substrate molecules to ADP. 2. oxidative phosphorylation – use of ATP synthase and energy derived from a proton (H+) gradient to make ATP, occurs only in O2 presence. The complete oxidation of glucose proceeds in stages: 1. glycolysis 2. pyruvate oxidation 3. Krebs cycle 4. electron transport chain & chemiosmosis For glycolysis to continue, NADH must be recycled to NAD+ by: 1. Fermentation – occurs when oxygen is not available; an organic molecule is the final electron acceptor. 2. Aerobic respiration – occurs when oxygen is available as the final electron acceptor. The fate of pyruvate depends on oxygen availability. When oxygen is present, pyruvate is oxidized to acetyl-CoA which enters the Krebs cycle. Without oxygen, pyruvate is reduced in order to oxidize NADH back to NAD+. Electron Transport Chain The electron transport chain (ETC) is a series of membrane-bound electron carriers. -embedded in the mitochondrial inner membrane -electrons from NADH and FADH2 are transferred to complexes of the ETC -each complex transfers the electrons to the next complex in the chain As the electrons are transferred, some electron energy is lost with each transfer This energy is used to pump protons (H+) across the membrane from the matrix inner membrane space. A proton gradient is established. The energy of the proton gradient is known as the chemiosmotic potential == proton motive force PMF. • Krebs cycle in the matrix, produces NADH and FADH2 • NADH and FADH2 transfers its hydrogens to the ETC, where they are split into H+ ions and electrons • The ETC acts as a H+ ion pump, pulling H+s into the intermembrane space • This causes intermembrane space pH to drop to 7 and raises matrix pH to 8 • electrons are conducted laterally through the membrane from one ETC complex to the next, each time donating some of their energy to "pump" H+ ions. • Finally the de-energized electrons are combined with hydrogen ions and oxygen to produce water in the matrix • This reaction is catalyzed by cytochrome oxidase • The rxn is inhibited by cyanide, which is why cyanide is a poison An overview of electron transport chain operations 2 mitochondrial compartments formed by 2 bilayer membranes. The light blue circles represent the (ETC), embedded in the membrane between the 2 compartments (the inner mitochondrial membrane) embedded in this membrane is the enzyme ATP synthase (pink) • These reactions store energy in a hydrogen gradient: H+ concentration is 10 X >>in intermembrane space than in the matrix • H+ diffuses back into the matrix through a channel in ATP synthase, producing an electric current • The shaft of the ATP synthase complex rotates (counterclockwise) when it conducts an electric current. • ATP is formed in the inner matrix: it must be transported across 2 membranes to get into the cytosol • Specific integral (intrinsic) membrane proteins accept electrons and H from NADH and FADH2 in the presence of O2. • NADH --> NAD+ – NADH from rxn in krebs and from the pyruvate dehydrogenase complex rxn. – The name of the protein/enzyme that oxidizes NADH is NADH reductase. – This enzyme dumps H+ into the intermembrane space. • FADH2 --> FAD – FADH2 from the succinate dehydrogenase rxn in Krebs. – The protein/enzyme that oxidizes FADH2 is succinate reductase. – This enzyme dumps H+ into the intermembrane space. Cytochrome oxidase is the name of the protein/enzyme which interacts with oxygen. • This enzyme dumps H+ into the intermembrane space. • Any chemical interferring with the exchange of electrons and protons between cytochrome oxidase and oxygen will halt the electron transport chain function and will cause respiration to stop. • Proteins in the inner mitochondrial membrane and cristae that have Fe, S, Cu as part of their structures are reduced and oxidized as electrons and H are moved along. • Each electron transport protein works in a one way direction. It 'grabs' electrons away from the previous component of the chain. • Protons dumped into the intermembrane space create a proton gradient across the inner mitochondrial membrane. The proton gradient is the stored energy that catalyzes the oxidative phosphorylation of ADP. • More ATP produced in oxidative phosphorylation vs anaerobic fermentation. Cytochrome oxidase NADH dehydrogenase Succinate dehydrogenase Ubiquinone Cytochrome c oxidoreductase Cyanide inhibits cytochrome oxidase • Comparing the Energy Capture of Glycolysis and Respiration • Most of the energy captured from the Krebs cycle is in the bonds of the coenzymes, NADH and FADH2 • In the electron transport chain the coenzyme energy is "cashed in" for ATP similar to currency exchange • The exchange rate different for different coenzymes – FADH2 gives 2 ATPs – NADH made in the mitochondria gets 3 ATPs – NADH made outside the mitochondria (in glycolysis) gets only 2 ATPs (some energy is lost in transporting this NADH into the mitochondria) The higher negative charge in the matrix attracts the protons (H+) back from the intermembrane space to the matrix. Most protons move back to the matrix through ATP synthase. ATP synthase is a membrane-bound enzyme that uses the energy of the proton gradient to synthesize ATP from ADP + Pi. theoretical energy yields - 36 ATP per glucose for eukaryotes Glycolysis 2 net ATP from substrate-level phosphorylation 2 NADH yields 6 ATP (assuming 3 ATP per NADH) by oxidative phosphorylation Transition Reaction 2 NADH yields 6 ATP (assuming 3 ATP per NADH) by oxidative phosphorylation Citric Acid Cycle 2 ATP from substrate-level phosphorylation 6 NADH yields 18 ATP (assuming 3 ATP /NADH) by oxidative phosphorylation 2 FADH2 yields 4 ATP (assuming 2 ATP /FADH2) by oxidative phosphorylation Total Theoretical Maximum Number of ATP Generated per Glucose in Prokaryotes 38 ATP: 4 from substrate-level phosphorylation; 34 from oxidative phosphorylation. In eukaryotic cells, the theoretical maximum yield of ATP generated per glucose is 36 to 38, depending on how the 2 NADH generated in the cytoplasm during glycolysis enter the mitochondria and whether the resulting yield is 2 or 3 ATP per NADH. • The NADH dehydrogenase of the inner mitochondrial membrane accept electrons only from NADH in the matrix. Problem: the inner membrane is not permeable to NADH, how can the NADH generated by glycolysis in the cytosol be reoxidized to NAD by O2 via the respiratory chain? Solution: Special shuttle systems carry reducing equivalents from cytosolic NADH into mitochondria by an indirect route. The most active NADH shuttle, which functions in liver, kidney, and heart mitochondria, is the malate-aspartate shuttle Malate Aspartate shuttle • In contrast to oxidation of mitochondrial NADH, cytosolic NADH, when it is oxidized via the electron transport system • if it proceeds via the malate aspartate shuttle gives rise to 3 ATPs • if it is oxidized by the glycerol phosphate shuttle gives rise to 2 equivalents of ATP Malate-Aspartate Shuttle Glycerol-3-phosphate shuttle : In skeletal muscles and Brain: