* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Macromolecules Notes
Size-exclusion chromatography wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Butyric acid wikipedia , lookup
Citric acid cycle wikipedia , lookup
Peptide synthesis wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Metalloprotein wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Genetic code wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Protein structure prediction wikipedia , lookup
Proteolysis wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
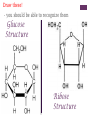
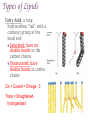
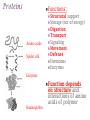
+Properties of Water – benefits for life Cohesion Adhesion Transparency Density Solvent Heat capacity + Macromolecules Notes + Organic Molecules All molecules containing carbon found in living systems with a few exceptions (CO2) Mostly have covalent bonds polar covalent bonds Non polar covalent bonds: Equal sharing, molecule is not charged. Note: Inorganic molecules are all other molecules. Can have ionic bonds like NaCl + PS! Things with carbon are organic UNLESS they are exclusively C & H (or CO2)… these are not organic. ie. Hydrogencarbonate : CH4 Macromolecules Polymer: a long molecule consisting of many similar or identical building blocks linked by covalent bonds Polymers Polymers are made up of monomers Monomers are small repeating units; the building blocks of polymers. Ex: Glucose is a monomer, starch is a polymer: many glucose bonded together make starch. Glucose Starch Condensation Reactions Condensation Reaction- building polymers Two molecules are joined to form a larger molecule, held by covalent bonds; requires an enzyme and produces one water molecule. Each monomer contributes to water that is made, one provides the -OH, one the -H. Aka dehydration reaction + Condensation Reaction For Example: Glucose + Galactose Lactose + water (monomer) + (monomer) (polymer) + water * Lactose is really called a dimer (only two monomers are bonded together) Di- means 2 ** Polymer is for many monomers bonded together; Poly- means many Hydrolysis How to break polymers into monomers bonds between monomers of a polymer are broken by the addition of water molecules; requires enzymes a H from water attaches to one monomer OH from water attaches to the other monomer + Hydrolysis For Example: Lactose + water Glucose + Galactose (polymer) + water (monomer) + (monomer) Classes of Macromolecules Carbohydrates Lipids Proteins Nucleic Acids Monosaccharides Monosaccharides: simplest carbohydrates simple sugars General formula (CH2O)n Major nutrients for cells raw material for other molecules disaccharides and polysaccharides Ex: glucose, fructose, galactose C6H12O6 Monosaccharides **Glucose: energy source carried by the blood to cells **Fructose: used to make fruit sweet tasting and attractive to animals Galactose: used to make milk Draw these! - you should be able to recognize them Glucose Structure Ribose Structure Disaccharides Disaccharides: two monosaccharides joined by a glycosidic linkage (covalent bond between monosaccharides using condensation) Ex: sucrose; maltose; lactose Disaccharides **Sucrose: glucose + fructose; carried by phloem to transport energy to cells in plants Maltose: 2 glucose; used in creating starch **Lactose: glucose + galactose; the sugar in milk; source of energy Polysacchrides Polysaccharides: storage and structural macromolecules made from a few hundred to a few thousand monosaccharides Ex: starch, glycogen, cellulose Storage Polysaccharides Starch: found in plants, polymer made of glucose molecules, used for energy **Glycogen: found in animals, a highly branched polymer of glucose (short term energy storage in liver and muscle cells) + Structural Polysaccharides **Cellulose: used to make strong fibers; major components on plant cell walls Bioweb.wku.edu Lipids Functions: Long term energy storage molecules in plants and animals Insulation Buoyancy Solids are known as fats; liquids are known as oils Animals: store fat Plants: store oils General Lipid Structure Glycerol attached to one or more fatty acids Fatty Acid Structue Fatty Acid: Draw this structure! Types of Lipids Fat: Composed of a fatty acid attached to glycerol Triglyceride: Consists of three fatty acids linked to glycerol by condensation reactions 02.03.17 How can you tell ribose and glucose apart? Today: - Wrap macromolecule notes - Option D Packet Begin Types of Lipids Fatty Acid: a long hydrocarbon “tail” with a carboxyl group at the head end Saturated: have no double bonds in the carbon chains Unsaturated: have double bonds in carbon chains Cis = Curved = Omega - 3 Trans = Straightened hydrogenated Types of Lipids Phospholipids: major components of cell membranes Hydrophilic head Two fatty acid tails (hydrophobic) Proteins Functions: Structural Amino acids Spider silk support Storage (not of energy) Digestion Transport Signaling Movement Defense Hormones Enzymes Enzymes Function Haemoglobin depends on structure and interactions of amino acids of polymer Proteins Made up of amino acids Amino acid chains form polypeptides, based on a specific sequence and vary in length from a few to thousands Proteins consist of one or more polypeptides folded and coiled into specific formations Proteins Denaturation (break down) of proteins is caused by: Change in pH Salt concentration Temperature Amino Acids Amino An Acid Structure: amino group bonded to a central carbon bonded to a carboxyl group, an “R” group (some other functional group) bonded to the central carbon Types of Amino Acids 20 different (don’t memorize) Grouped by the properties of side chain Non-polar side chains = hydrophobic Polar side chains = hydrophillic Identify the following Glucose Ribose Fatty Acid Amino acid + Nucleic Acids Types: DNA and RNA Made of repeating units of nucleotides Nucleotides created by a sugar, phosphate group and a nitrogen base. DNA contains deoxyribose sugar RNA contains ribose sugar 02.06.17 Describe the relationship between a monomer and polymer I can model protein folding and review macromolecules. Types of Lipids – Clarification/Correction!! Fatty Acid: a long hydrocarbon “tail” with a carboxyl group at the head end Saturated: have no double bonds in the carbon chains Unsaturated: have double bonds in carbon chains Cis = Curved = Omega - 3 Trans = Straightened - hydrogenated These are both types of unsaturated fat! All about where the hydrogens are in the double bond. Cis – liquid at room temperature Trans – solid at room temp Modeling Polypeptide Chains Look at your card (or help your neighbor with theirs) What properties does your amino acid have? Protein Folding Our sequence: Met, Lys, His, Val, Ser, Leu, Asp, Glu, Cys, Asn, Tyr, Val, Phe, Trp, Pro, Ser, Thr, Gln, Cys, Gly Hold your card in your left hand. Side chain (R) Touch the right shoulder of the person in front of you with your right hand. Peptide bond. Check your card Key! Blue – hydrophilic Red – hydrophobic Yellow Black – cysteine – acidic (negatively charged) Purple – basic (positively charged) Protein Folding – Don’t break the peptide bond!! N-terminus n Met n + Lys + + His + n Val n p Ser p Hallway! n Leu n n First bond +is cysteine + - + + - - Asp n Disulfide Bridge - - Glu - S Cys S p Asn S p n Val n n - Then charged interactions n n S S pThen WATER! p p n n n S S n n p n n (c) n Hydrophobic Interactions (d) p Ser p Gln S Cys n Gly C-terminus (a) p p n n Pro p Thr p n n p p n Trp (b) n p p p p n n Phe n n Ionic Bonding p p Tyr p p Figure 2. Steps in the protein folding demonstration. Each circle represents an amino acid. The symbols used within the circles indicate the grouping to which the amino belongs: n, nonpolar; p, polar; -, acidic; +, basic; S, sulfur containing cysteine. (a) Primary structure of the polypeptide. (b) Configuration following the formation of the covalently bonded disulfide bridge. (c) Configuration with ionic bonding between acidic and basic side groups. (d) Final form of protein with hydrophobic interactions included. What you did. n Disulfide bridge! React to Water! + + n p Ionic Bonding n + Disulfide + Bridge n p n n Disulfide Bridge p - n S S p S n p p n n p n p p p p n n n n (b) n n p + - n + p n p p -n+ nS- Ionic Bonding p p n n n SS + S + n p S n p p pp nn n p n n n n + - p p p n n (c) n n + - - + - nn n S pp n n SS S - n S S n p n n p np n p p p n n p n p p n p Hydrophobic p Interactions Hydrophobic Hydrophobic Interactions Interactions (d) p p p n p p nn p n n + - Ionic + Ionic n Bonding pS Bonding p S p pp n n S - n + n n + n Disulfide n Bridge - p (d) n n Practice! Work independently or with a small group to review your macromolecule knowledge This is a homework assignment that will be checked during class tomorrow.