* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Basics of Cell Culture
Cytokinesis wikipedia , lookup
Programmed cell death wikipedia , lookup
Cell growth wikipedia , lookup
Extracellular matrix wikipedia , lookup
Cell encapsulation wikipedia , lookup
Cellular differentiation wikipedia , lookup
List of types of proteins wikipedia , lookup
Tissue engineering wikipedia , lookup
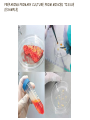
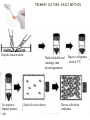
INTRODUCTIONTO CULTURE CELL C O M PA R I N G O R G A N , T I S S U E A N D C E L L C U LT U R E S TISSUE, CELL AND ORGAN CULTURE-1 • Tissue culture Technology is known as the general term to describe the in vitro cultivation of organs, tissues & cells at defined temperature using an incubator, supplemented with a medium containing cell nutrients & growth factors. • Tissue culture : the small tissue fragments are explanted into a suitable medium and encouraged to grow in isolation, to form colonies, and possibly to continue some of their normal functions. • Cell culture : the cells of a tissue, or even individual cells, are made to grow in a special medium. – Sub culturing of primary cells leads to the generation of cell lines (contain one type of cells) – Lineage of cells originating from the primary culture is called a cell strain (forming a cell line) – Cell lines have limited life span, they passage several times before they become senescent. • Organ culture : growth is only of minor interest, but embryological development and the maintenance of normal physiological functions are the chief aim and object. TISSUE, CELL AND ORGAN CULTURE-2 • Different types of cells grown in culture includes fibroblasts, skeletal tissue, cardiac, epithelial tissue (liver, breast, skin, kidney) and many types of tumor cells. • Cells when surgically or enzymatically removed from an organism and placed in suitable culture environment will grow are called as primary culture where the Primary cells have a finite life span – they stop growing and ultimately die after only a few subculturing steps – contains heterogeneous population of cells (different types of cells present) since it is directly originated from the tissue. – Cells such as macrophages and neurons do not divide in vitro so can be used as primary cultures • After obtaining the culture primary cell culture, then the irrelevant cells are discarded. The dominant cells that multiply and grow in uniform populations are of interest and used for cell cultures. CELL CULTURES ACCORDING TO THEIR BASIC SOURCES AND CULTURING CONDITIONS Basically we can summarize cell culture types as following: • Primary cell cultures: cells were taken directly from tissues – Explant cultures (after tissue dissection) – Enzymatic dissociation culture (tissue dissociated after enzyme digestion) • Secondary Cell Cultures: derived from prinmary cultures to form a cell line – Monolayer cultures (anchorage dependent cultures, that are adherent cell cultures, such as epithelial cells of intestine) – Suspension Culture (non anchorage dependent cultures, such as blood cells) VARIOUS APPLICATIONS OF TISSUE CULTURE TECHNIQUES GENERAL CULTURE PARAMETERS-1 • Culture volume is the factor determined by the purpose of the technology used. Such that: 1. Small scale culture of cells (up to 1L) are good to establish cell culture • for studying cell morphology • for comparing the effects of agents on growth and metabolism, etc 2. Animal cell culture is a widely used production process in biotechnology, with systems in operation at scales over 10000 liters • To produce vaccine material • To isolate cell constituent (DNA, enzymes, proteins,…), etc. Cell Sources: • The list of different cell types which can be grown in culture is extensive. For applications the commonly used cells and regularly updated list of commercially available cell stocks are available through : • ATCC AmericanType Culture Collection (American) https://www.lgcstandards-atcc.org/Products/Cells_and_Microorganisms/Cell_Lines.aspx?geo_country=tr • The European Collection of Cell Cultures (British) https://www.phe-culturecollections.org.uk/collections/ecacc.aspx GENERAL CULTURE PARAMETERS -2 C E L L C U L T U R E C O L L E C T I ONS ( A V A I L A B L E S O U R C ES ) . GENERAL METHODS AND CULTURE PARAMETERS -2 P R I M A RY C U L T U R E • Cells when surgically or enzymatically removed from an organism and placed in suitable culture environment will attach and grow are called as primary culture where the Primary cells have a finite life span. • Primary culture contains a very heterogeneous population of cells (different types of cells present) • Cells such as macrophages and neurons do not divide in vitro so can be used as primary cultures PRIMARY CULTURE (EXAMPLE) • Stage of culture after the cells are isolated from the tissue and proliferated under the appropriate conditions until they completely occupy the substrate • Monolayer – Reach Confluence • Then need to be subcultured (Passaged) • Split & Move to fresh medium, new vessels Chinese Hamster Ovary Cells (CHO) PREPARING PRIMARY CULTURE FROM MINCED TISSUE (EXAMPLE) PRIMARY CULTURE: BASIC METHOD Chop the tissue in media Use trypsin to Separate primary cells Wash with buffer and centrifuge, then discard supernatant Seed cells at low density Digest w collagenase / incub @37ºC Harvest cells before confluence ISOLATION OF CELLS FOR CULTURING ADVANTAGES OF CELL CULTURE COMPARED TO ANIMAL STUDIES AND ORGAN CULTURES: • Contains disintegrated tissue or primary cell lines • Requires less effort and time frame to perform task • Easy to perform biochemiscal molecular, immunological and cytological assays • No histological characterization required • No biochemical differentiation is possible (loss of such differentiation) • Propogation is possible • Intersample variation is very low • Possible to study in high quantities. LIMITATIONS OF CELL CULTURE APPLICATIONS • Cell type identification is a tedious and difficult process – If obtained from a primary cell line, or somehow if the cell type is confused with the others in cell stock tank, the cell type identification is a must thing to do. Such process requires variety of tests including genetic and surface marker analysis and is not easy to handle • Markers specific for a certain cell type may not be expressed – During adaptation to culture conditions some of the properties of cell is missing such as surface receptors, factors and some properties. So researcher must be careful about such changes while ordering the cells or producing the cells from primary sources. • Histology is difficult to create – Most of the cells in culture conditions differentiate such that they look alike. This is easily oberved with primary cell cultures that contains heteregenous mixtures of cells but look similar under light microscope. LIMITATIONS OF CELL CULTURE APPLICATIONS • Cells are subject to chemical and bacterial contamination – Culturing environment is free of immune defense and hence with less careful handling may cause bacterial or mycosis contaminations. Also handling of treatment agents (drugs, factors etc) and media to supplement cells may cause chamical contamination, that is overloading of cells with some chemical agents. • Use of Strict aseptic procedures – Laboratory should provide environment for aseptic work, equipped properly and also the lab workers must be trained for clean lab work procedures. These are time and Money consuming steps and every work places may not compliant with such conditions. • Budget restrictions – The use of disposable labware is costly to many labs. – The supplements used for culture media including reagents are expensive – The waste disposal charges are high (all cell lab wastes are treated as bioh • Cultures may represent genetic and phenotypic instability – cultures are made of continuous cell lines but they are not EXACTLY immortal in nature. To protect them every set of cells should be frozen to store and hence passaging number can be kept low. – Passaging (subculturing of a cell sample) has certain limits to maintain genetic and phenotypic properties. – High order of passaging may cause loss of genetic and phenotypic properties. COMPARING ORGAN AND CELL CULTURES • Cell cultures , in nature, differ from organ cultures in a way that: – .they do not regain 3D geometry – They require substrates to grow on (certain surfaces to interact with to induce growth signal) – They do not have specific cell interactions attributed to the tissue of origin – Cells proliferate: in tissues the cell number is limited and controlled by signaling and genetic material. However in culture cells do not have such limitations. They grow in logarithmic numbers and limitation is the nutricional supplement. – Cells are lack of systemic components that are necessary for the regulation of homeostasis. – Energy metabolism is not highly regulated in cell cultures. ADVANTAGES OF CELL CULTURING • Results are consistent and reproducible • Statistical analysis of variance is reduced • Physiological conditions are under control • Selective media can be changed and help to control cell line • Whenever requires some sample of cells can be stored (cryopreservation) • Cells can be characterized easily by immunostaining • Contamination can be controlled by Use of appropriate aseptic techniques • Small scale study helps to reduce the amount of reagents • Several critical parameters, such as time, döşe and concentration , can be easily controlled • Robotic systems and microtitration supplies help to reduce time and personal effort for large sets of studies or large scale studies including production of bioagents.